Ultra-fast HPM Detectors Improve NAD(P)H FLIM
Wolfgang Becker, Lukas Braun, Becker &
Hickl GmbH
Abstract: Metabolic imaging by NAD(P)H FLIM
requires the decay functions in the individual pixels to be resolved into the
decay components of bound and unbound NAD(P)H. Metabolic information is
contained in the lifetime and relative amplitudes of the components. The
separation of the decay components and the accuracy of the amplitudes and
lifetimes improves substantially by using the ultra-fast HPM-100-06 and HPM-100-07
hybrid detectors. The IRF width in combination with the SPC-150N and SPC-150NX
TCSPC modules is less than 20 ps [1]. An IRF this fast does not interfere
with the fluorescence decay. The usual deconvolution process in the data
analysis then virtually becomes a simple curve fitting, and the decay
parameters are obtained at unprecedented accuracy.
Metabolic Imaging
NADH and its phosphorylated form, NADPH,
are natural coenzymes that are involved in the energy production of the cell. The
reduced forms of NADH and NADPH are fluorescent. It is known that the
fluorescence lifetimes depend on the binding to proteins [13, 16]. The
bound-NADH lifetime is in the range of 1.2 ns to 4 ns, the
unbound-NADH lifetime in the range from about 300 ps to 500 ps. For
NADPH the situation is similar, with slightly different lifetimes of the
components [6]. The concentration ratio of bound and unbound NADH depends on
the type of the metabolism: When the cell is in the state of oxidative
phosphorylation the bound/unbound ratio is higher than in the state of
glycolysis. Consequently, the relative amplitudes of the decay components
change with the type of metabolism, and so does the mean fluorescence lifetime.
Since normal cells are running preferentially oxydative phosphorylation while
tumor cells are running glycolysis the corresponding changes in the
fluorescence decay parameters bear the potential to distinguish between healthy
cells and tumor cells [5, 15, 19, 20, 21, 24].
Changes in the NADH decay parameters are also observed during maturation of
stem cells, during hypoxia, and during infection, during wound healing [3, 9, 10,
11, 12, 14, 18,]. The NADH
lifetime in combination with the NADH/FAD redox ratio [8] has been used to predict drug response in breast cancer [22, 23].
Please see also [3] and [4] for a summary.
NADH FLIM with Fast Detectors
In principle, FLIM of NAD(P)H is possible
with all bh FLIM systems. NAD(P)H can be excited by one-photon excitation at a wavelength
of 375 nm or shorter, or by two-photon excitation in the range of 720 nm
to about 780 nm. Two-photon excitation is usually preferred because it is
considered less invasive to the cells (though this has never been directly
proved). In any case, two photon excitation has the advantage that it
penetrates much deeper into tissue, and that it has no problems with optical
aberrations of the microscope optics in the UV. Another advantage of two-photon
excitation is that the pulse width of the femtosecond laser does not contribute
to the temporal instrument-response function (IRF) of the TCSPC FLIM system. The
system therefore delivers the shortest possible IRF for a given detector‑TCSPC
combination. Typical IRF widths are 120 ps for GaAsP hybrid detectors,
250 ps for fast conventional PMTs, and about 300 ps for conventional
PMTs with GaAsP cathodes [3]. This is not much faster than the dominating decay
time of the unbound NADH, which is about 300 ps [6]. It can therefore be
expected hat faster detectors improve the accuracy of the fluorescence-decay
analysis of NADH FLIM. However, there is a problem. The GaAsP cathode of the
typical high-efficiency FLIM detectors limits the speed to about 120 ps. Faster
detectors either have extremely small active areas (SPADS) and are thus not
applicable to NDD detection in two-photon microscopes, or they have
conventional photocathodes with low quantum efficiency (PMTs). A possible
compromise are the new Hamamatsu R10467-06 and -07 hybrid detectors with high-efficiency
bi-alkali and multi-alkali cathodes. Although the photocathodes do not reach
the quantum efficiency of a GaAsP cathode the hybrid detector principle makes
up for a part of the loss: Unlike a conventional PMT a hybrid detector has no
loss of photoelectrons at the first dynode. Virtually all photoelectrons that
leave the photocathode also cause a pulse at the output of the detector. We have
shown recently that the HPM-100-06 and -07 detectors (based on the R10467-06
and -07) deliver an IRF width of less than 20 ps when operated with
SPC-150N, SPC-150NX or SPC‑180NX TCSPC modules [1]. An IRF this fast does
not interfere with the fluorescence decay. The usual deconvolution process in
the data analysis [3] then virtually becomes a simple curve fitting. It can
therefore be expected that the high time resolution delivers a better photon efficiency
in the data analysis and thus makes up for the lower sensitivity.
Results
For recording the data shown below we used
a Zeiss LSM 880 NLO multiphoton microscope with an HPM‑100‑06
and an HPM-100-07 attached to the NDD output via the usual Zeiss NDD T adapter
[2]. The signals were recorded by a Simple-Tau 152 system with two SPC‑150N
TCSPC modules. The system is able to record in two wavelength channels in
parallel, the images shown here are from a wavelength channel from 440 nm
to 480 nm. The excitation wavelength was 740 nm. The data were
recorded with 512 x 512 pixels and 1024 time channels per pixel. The time
channel width was 10 ps.
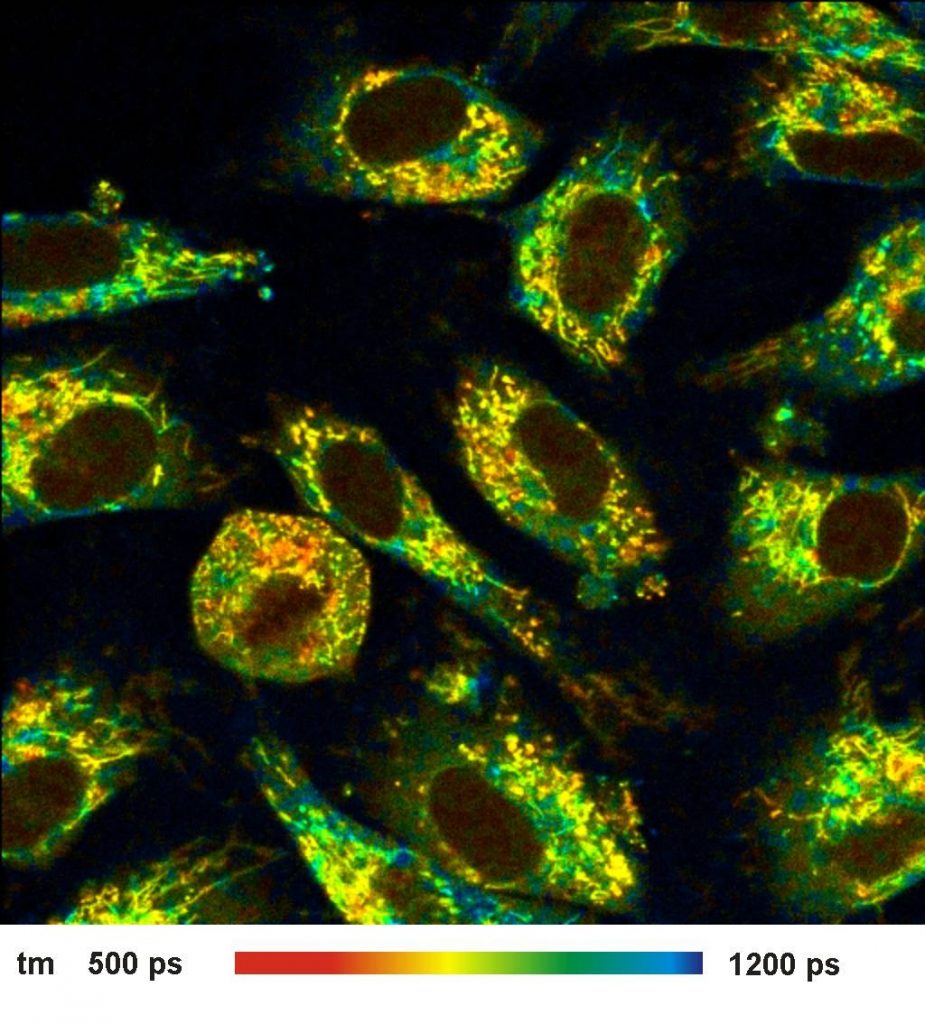
A lifetime image of the amplitude-weighted
lifetime of a double-exponential fit is shown in Fig. 1, left. Decay data in a
selected spot of 9x9 pixels on the right. As expected, the time resolution of
the detection system is excellent. The rise of the fluorescence occurs over
less than two time channels, indicating that the IRF is indeed shorter than
20 ps.
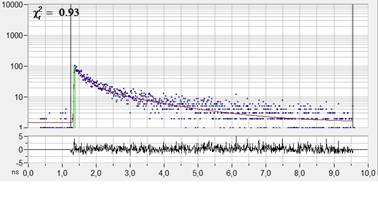
Fig. 1: Left: NADH Lifetime image, amplitude-weighted lifetime of
double-exponential fit. Right: Decay curve in selected spot, 9x9 pixel area.
FLIM data format 512x512 pixels, 1024 time channels. Time-channel width 10ps.
Images of the amplitude ratio, a1/a2
(unbound/bound ratio), and of the fast (t1, unbound NADH) and the slow decay
component (t2, bound NADH) are shown in Fig. 2. Such images are normally noisy,
and visibly contain fitting artefacts. Not so in the data recorded with the
fast detectors and SPC modules. Due to the near-ideal temporal resolution the
FLIM data analysis delivers the decay components at extremely high precision,
and the images are free of fitting noise and fitting artefacts.
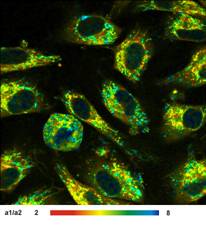
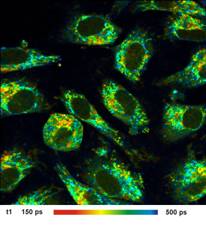
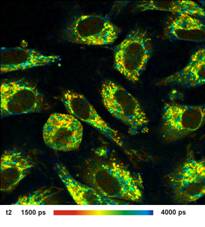
Fig. 2: Left to right: Images of the amplitude ratio, a1/a2 (unbound/bound
ratio), and of the fast (t1, unbound NADH) and the slow decay component (t2, bound
NADH). FLIM data format 512x512 pixels, 1024 time channels. Time-channel width
10ps.
Conclusion
The ultra-fast HPM-100-06 and HPM-100-07
hybrid detectors in combination with the SPC-150N TCSPC modules improve the
accuracy of NAD(P)H FLIM dramatically. The IRF is so fast that it does no
longer interfere with the decay times of the fast fluorescence components. The
deconvolution process in the data analysis therefore virtually becomes a simple
curve fitting, and the decay parameters are obtained at unprecedented accuracy.
Even the a1/a2 images and the t1 and t2
images are virtually free of noise or fitting artefacts.
Acknowledgements
The data shown in this application note
were recorded at the 2nd International Workshop on Advanced Time-Resolved
Imaging Techniques at BioCev, Vestec near Prague , May 16-17, 2017. We thank
Dr. Ale Benda of BioCev for providing the LSM 880 NLO and Dr. Ondrej ebasta of
Charles University for providing his Simple Tau system and the NDD T adapter for
the experiments.
References
1.
Becker & Hickl GmbH, Sub-20ps IRF Width from
Hybrid Detectors and MCP-PMTs. Application note, available on
www.becker-hickl.com
2.
Becker & Hickl GmbH , Modular FLIM Systems
for ZeissLSM 710 / 780 / 880 Family Laser Scanning Microscopes. User
Handbook, available on www.becker-hickl.com
3.
W. Becker, The bh TCSPC handbook. Becker &
Hickl GmbH, 9th ed. (2021). Available on www.becker-hickl.com
4.
W. Becker (ed.) Advanced time-correlated single
photon counting applications. Springer, Berlin, Heidelberg, New York (2015)
5.
D.K. Bird , L. Yan , K. M. Vrotsos , K. E.
Eliceiri , E. M. Vaughan. Metabolic mapping of MCF10A human breast cells via
multiphoton fluorescence lifetime imaging of coenzyme NADH. Cancer Res
65:87668773 (2005)
6.
T. S. Blacker, Z. F. Mann, J. E. Gale, M.
Ziegler, A. J. Bain, G. Szabadkai, M. R. Duchen, Separating NADH and NADPH
fluorescence in live cells and tissues using FLIM. Nature Communications 5,
3936-1 to -6 (2014)
7.
T. Y. Buryakina, P.-T. Su, W.J. Syu, C.A. Chang, H.-F. Fan, F.-J. Kao, Metabolism of HeLa cells revealed through
autofluorescence lifetime upon infection with enterohemorrhagic eschericha
coli. J. Biomed. Opt. 17(10) 101503-1 to -9
8.
B. Chance, Pyridine nucleotide as an indicator
of the oxygen requirements for energy-linked functions of mitochondria. Circ
Res 38, I31I38 (1976)
9.
G. Deka, W.W. Wu, F.-J. Kao, In vivo wound
healing diagnosis with second harmonic and fluorescence lifetime imaging. J.
Biomed. Opt. 18(6), 061222-1 to -8
10.
U. Gehlsen, A. Oetke, M. Szaszak, N. Koop, F.
Paulsen, Andreas Gebert, G. Huettmann, P. Steven, Two-photon fluorescence
lifetime imaging monitors metabolic changes during wound healing of corneal
epithelial cells in vitro. Graefes Arch. Clin. Exp. Ophthalmol 250, 1293-1302
(2012)
11.
S. Kalinina, V. Shcheslavskiy, W. Becker, J.
Breymayer, P. Schäfer, A. Rück, Correlative NAD(P)H-FLIM and oxygen
sensing-PLIM for metabolic mapping. J. Biophotonics 9(8):800-811 (2016)
12.
K. König, A. Uchugonova, E. Gorjup, Multiphoton
Fluorescence Lifetime Imaging of 3D-Stem Cell Spheroids During Differentiation.
Microsc. Res. Techn. 74, 9-17(2011)
13.
J.R. Lakowicz, H. Szmacinski, K. Nowaczyk, M.L.
Johnson, Fluorescence lifetime imaging of free and protein-bound NADH, PNAS 89,
1271-1275 (1992)
14.
A. V. Meleshina, V. V. Dudenkova, M. V.
Shirmanova, V. I. Shcheslavskiy, W. Becker, A. S. Bystrova, E. I. CherkasovaE.
V. Zagaynova, Probing metabolic states of differentiating stem cells
usingtwo-photon FLIM. Scientific Reports 6:21853 (2016)
15.
M.V. Shirmanova, V.I. Shcheslavskiy, M.M.
Lukina, W. Becker, E.V. Zagaynova, Exploring tumor metabolism with
time-resolved fluorescence methods: from single cells to a whole tumor. In: V.
Tuchin, J. Popp, V. Zakharov, Multimodal Optical Diagnostics of Cancer.
Springer (2020)
16.
R.J. Paul, H. Schneckenburger, Oxygen concentration
and the oxidation-reduction state of yeast: Determination of free/bound NADH
and flavins by time-resolved spectroscopy, Naturwissenschaften 83, 32-35
(1996)
17.
A. Heikal, Intracellular coenzymes as natural
biomarkers for metabolic activities and mitochondrial anomalies. Biomark. Med.
4(2), 241263 (2010)
18.
M. Szaszak, P. Steven, K. Shima, R.
Orzekowsky-Schröder, Gereon Hüttmann, I.R. König, W. Solbach, J. Rupp,
Fluorescence Lifetime Imaging Unravels C. trachomatis Metabolism and Its
Crosstalk with the Host Cell. PLOS Pathogens 7, e1002108-1 to 12 (2011)
19.
M. C. Skala, K. M. Riching, D. K. Bird, A.
Dendron-Fitzpatrick, J. Eickhoff, K. W. Eliceiri, P. J. Keely, N. Ramanujam, In
vivo multiphoton fluorescence lifetime imaging of protein-bound and free
nicotinamide adenine dinucleotide in normal and precancerous epithelia. J.
Biomed. Opt. 12 02401-1 to 10 (2007)
20.
M. C. Skala, K. M. Riching, A.
Gendron-Fitzpatrick, J. Eickhoff, K. W. Eliceiri, J. G. White, N. Ramanujam, In
vivo multiphoton microscopy of NADH and FAD redox states, fluorescence
lifetimes, and cellular morphology in precancerous epithelia, PNAS 104,
19494-19499 (2007)
21.
R. Suarez-Ibarrola, L. Braun, P. Fabian
Pohlmann, W. Becker, A. Bergmann, C. Gratzke, A. Miernik, K. Wilhelm, Metabolic
Imaging of Urothelial Carcinoma by Simultaneous Autofluorescence Lifetime
Imaging (FLIM) of NAD(P)H and FAD. Clinical Genitourinary Cancer (2020)
22.
A. J. Walsh, R. S. Cook, H. C. Manning, D. J.
Hicks, A. Lafontant, C. L. Arteaga, M. C. Skala, Optical Metabolic Imaging Identifies
Glycolytic Levels, Subtypes, and Early-Treatment Response in Breast Cancer.
Cancer Res. 73, 6164-6174 (2013)
23.
A. J. Walsh, R. S. Cook, M. E. Sanders, L.
Aurisicchio, G. Ciliberto, C. L. Arteaga, M. C. Skala, Quantitative Optical
Imaging of Primary Tumor Organoid Metabolism Predicts Drug Response in Breast
Cancer. Cancer Res 74, OF1-OF11 (2014)
24.
Q. Yu, A. A. Heikal, Two-photon autofluorescence
dynamics imaging reveals sensitivity of intracellular NADH concentration and
conformation to cell physiology at the single-cell level. J. Photochem. Photobiol.
B 95, 46-57 (2009)
Contact:
Wolfgang Becker, Becker & Hickl GmbH, Berlin,
Germany. Email: becker@becker-hickl.com