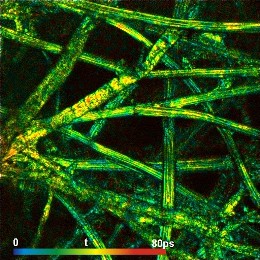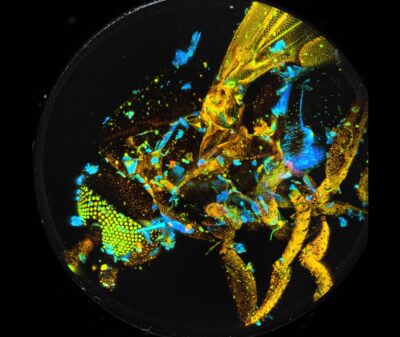

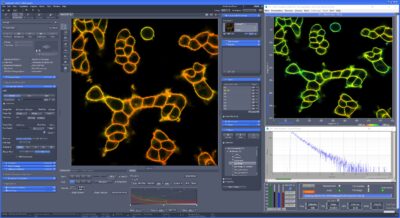
New FLIM Systems For Zeiss LSM 980 Microscopes
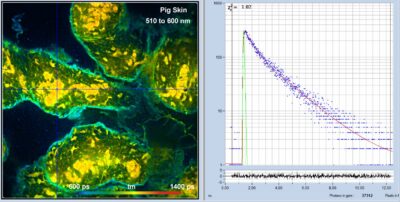
Two-Photon FLIM with a Small Femtosecond Fibre Laser
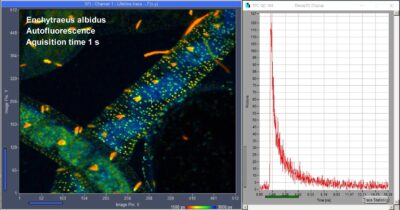
SPC-QC-104 – Precision FLIM and Fast FLIM in One

High-Resolution LIDAR with the SPC-QC-104
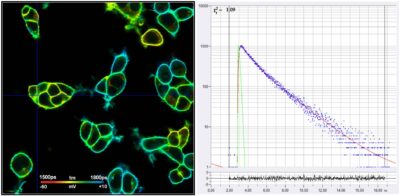
FLIM Records Membrane Potentials in Cells
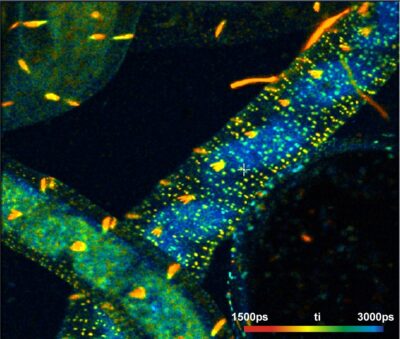
bh Express-FLIM System Runs at Near-Video Speed
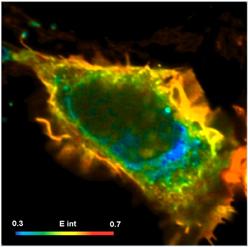
New FLIM-FRET Technique is Free of Calibration
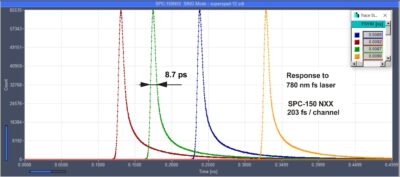
SPC-150NXX TCSPC system with ultra-fast SPAD delivers 8.7 ps IRF width
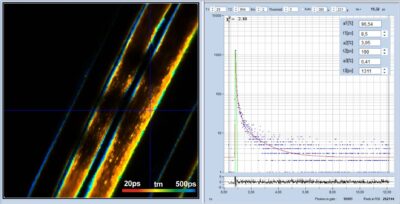
High-Resolution Multiphoton FLIM Reveals Ultra-Fast Fluorescence Decay in Human Hair
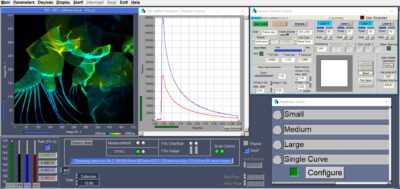
Record FLIM at Unprecedented Precision
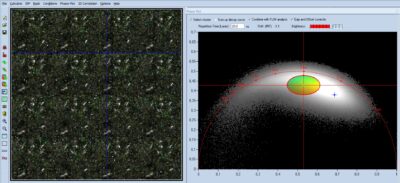
Label-Free Multiphoton FLIM of Moving Bacteria
Metabolic FLIM of Tumors with DCS-MACRO System
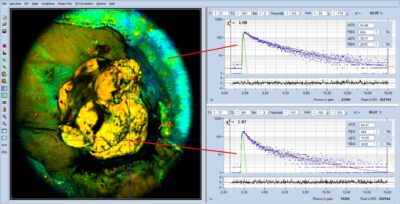
Ultra-Fast Fluorescence Decay in Malignant Melanoma
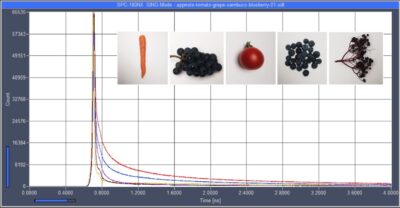
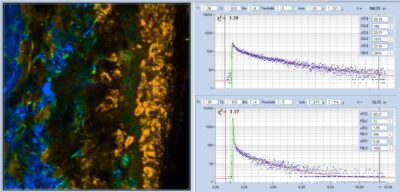
Ultra-Fast Fluorescence Decay in Natural Carotenoids
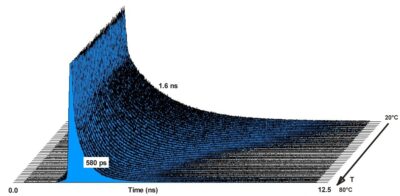
Micro-Volume Temperature Measurement by TCSPC

SPC-QC-104 Three-Channel TCSPC / FLIM Module Released
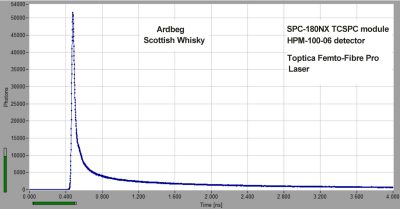
Ultra-Fast Fluorescence Decay in Scottish Whisky

USB-Controlled Picosecond/CW Diode Lasers
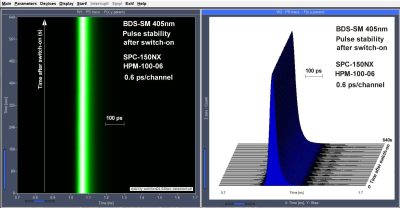
Sub-4ps Timing Stability of BDS-SM Series ps Diode Lasers
SPC-180NXX Records FLIM at 300 Femtoseconds / Channel

Ultra-Fast Version of SPC-180 TCSPC / FLIM Module Available

9th Edition of the bh TCSPC Handbook Available for Download
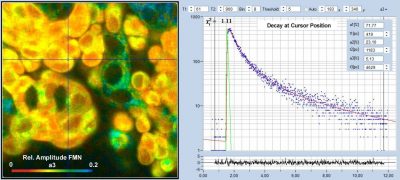
DCS-120 FLIM System Detects FMN in Live Cells
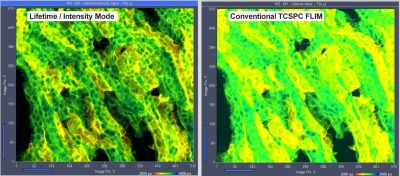
Lifetime-Intensity Mode Delivers Better FLIM Images
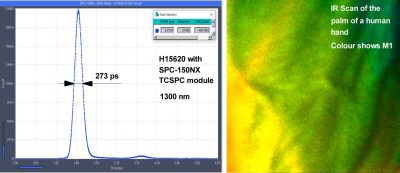
273 ps FWHM TCSPC Response with Hamamatsu H15620 NIR PMT
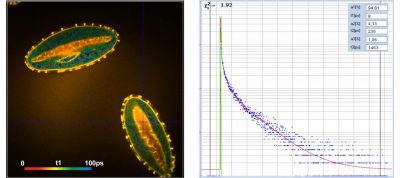
Two-Photon FLIM of Pollen Grains Reveals Ultra-Fast Decay Component
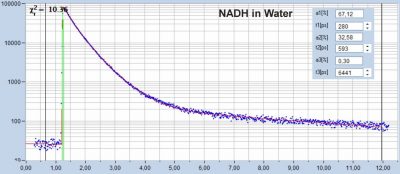
High-Resolution Measurement of NADH and FAD Fluorescence Decay with DCS‑120 MP System: 19 ps IRF Width
Now Available: Bigger and Better Photons – The Road to Great FLIM Results. Educational Brochure for FLIM Users

Ultra-Fast TCSPC / FLIM Module with PCIe Interface

Next Generation DCS-120 FLIM Systems
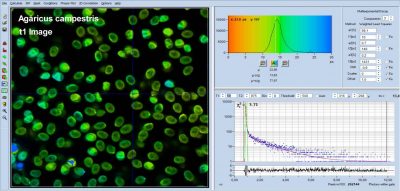
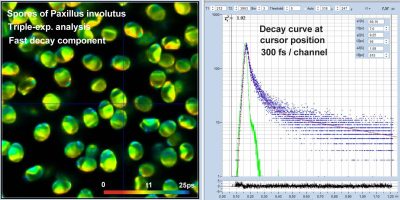
bh FLIM Detects Ultra-Fast Decay in Mushroom Spores
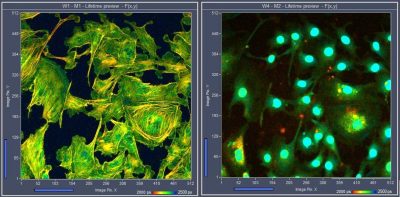
Excitation-Multiplexed FLIM Detects Multiple Fluorophores
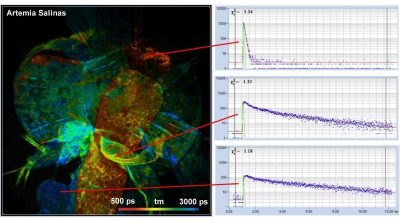
Two-Photon FLIM System with a Femtosecond Fibre Laser

Four ps Diode Lasers – One Fibre Output
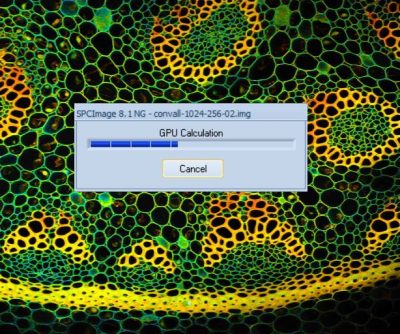
SPCImage NG FLIM Analysis Runs GPU Processing
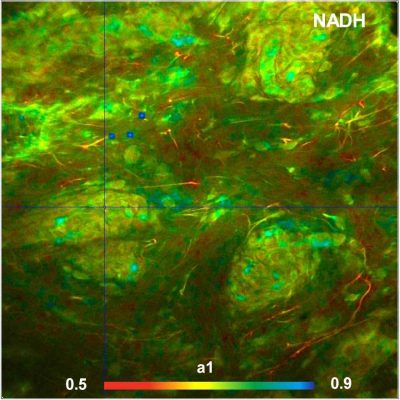
Two-Photon Metabolic-FLIM System Records NAD(P)H and FAD Data Simultaneously
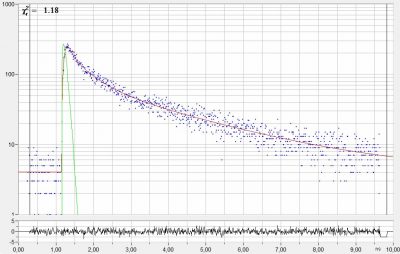
Modelling of IRF Makes IRF Recording Unnecessary
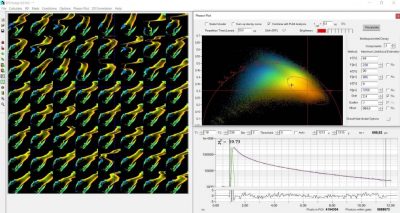
Mosaic FLIM records precision lifetime data of a moving object
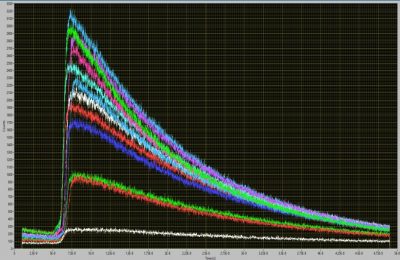
bh Release Max-Tau 12-Channel TCSPC System
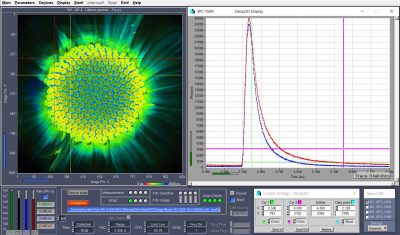
New Version of SPCM Software Available
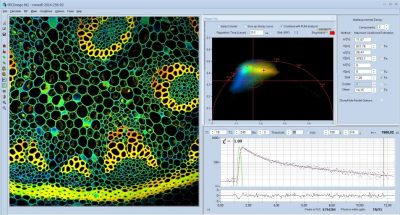
Next Generation SPCImage FLIM Analysis Software
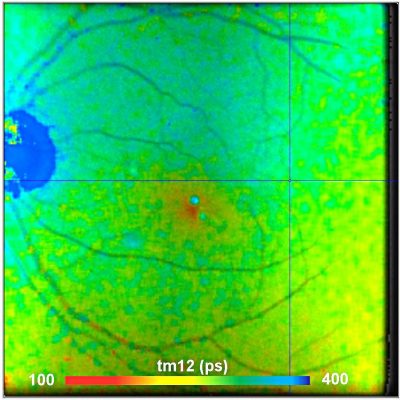
Shifted-Component Model Improves FLIO Data Analysis
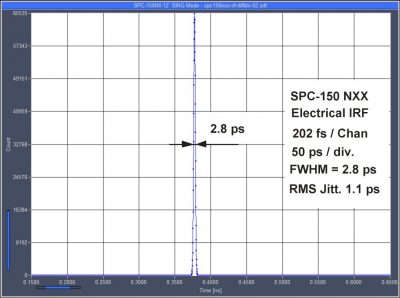
New TCSPC module has < 1.1 ps timing jitter
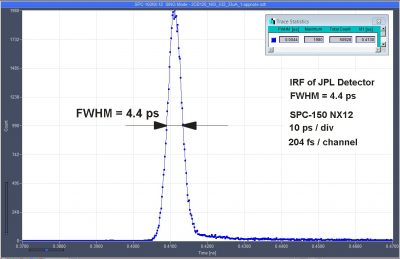
4.4 ps IRF with NbN Superconducting Nanowire Detector
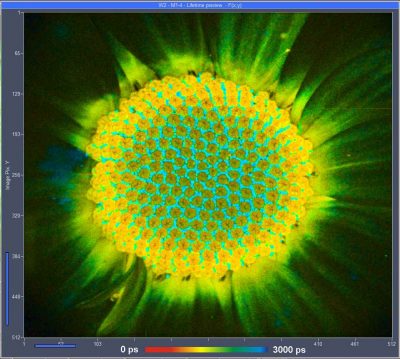
DCS-120 MACRO System Runs Fast FLIM
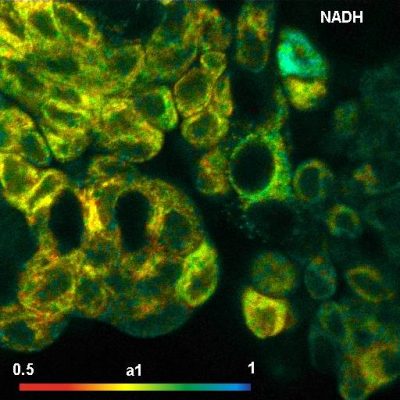
DCS-120 System Runs Metabolic FLIM
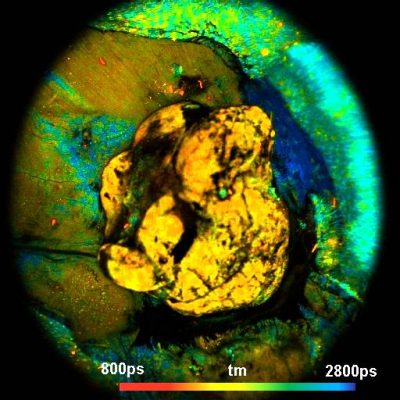
DCS-120 MACRO FLIM system detects tumors in mice
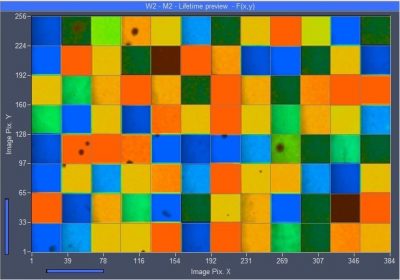
DCS-120 System Scans Well Plates

TCSPC System Records FLIM of a Rotating Object

New SPCM Version Comes With New Software Functions
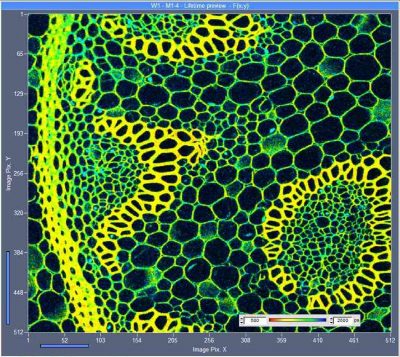
Fast-Aquisition Multiphoton FLIM with the Zeiss LSM 880 NLO

Advanced Time-Correlated Single Photon Counting Applications’ among Springer’s 15% of best performing books
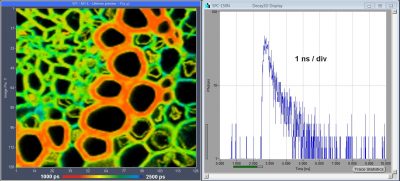
Fast-Aquisition FLIM System with 25 ps IRF Width
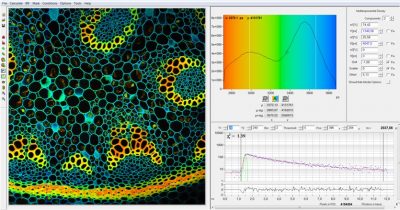
SPCImage Comes with New User Interface

SPCM Data Acquisition Software Controls Ti:Sa Lasers and AOM
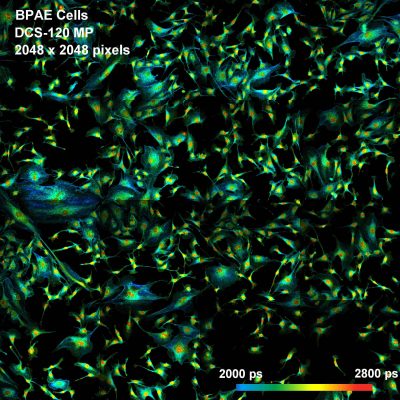
DCS-120 FLIM system records X-Y mosaics
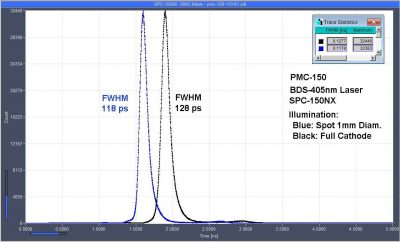
New PMC-150 detector has <120 ps IRF width
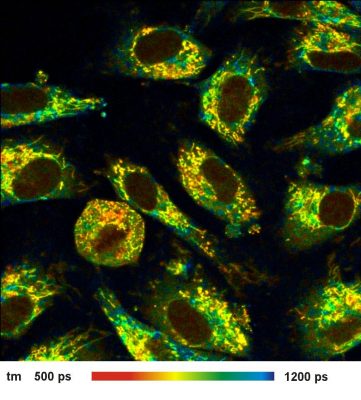
New ultra-fast detectors improve NADH FLIM
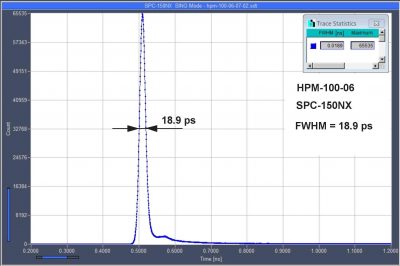
Sub-20 ps IRF Width from Hybrid Detectors and MCP PMTs
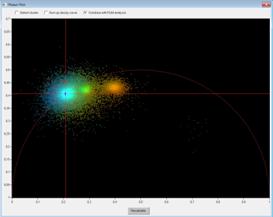
SPCImage Combines Time-Domain FLIM Analysis with Phasor Plot
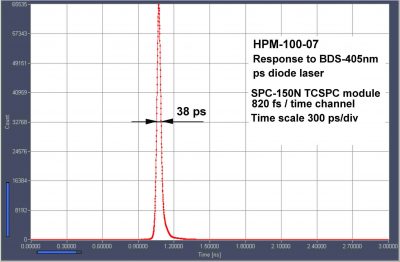
bh Introduce Ultra-Fast Hybrid Detector

Full Set of TCSPC / FLIM Cards with PCI Express Interface available
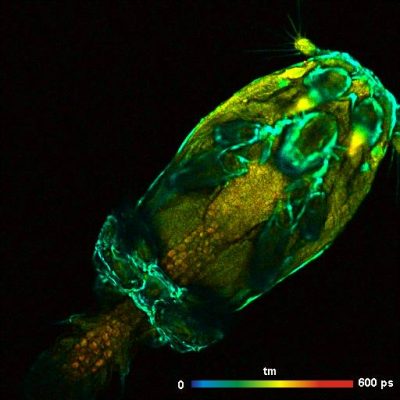
bh TCSPC Systems Record FLIM with Sutter MOM Microscopes
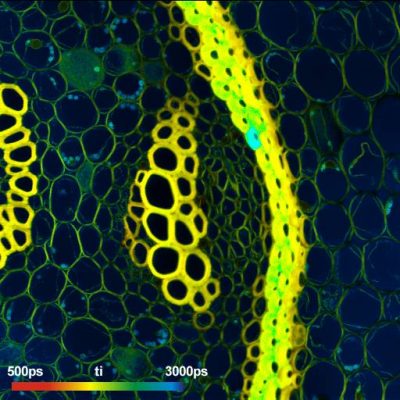
SPC Modules Record TCSPC FLIM with Bidirectional Scanning

New SPC-160PCIE TCSPC / FLIM module has PCI Express Interface
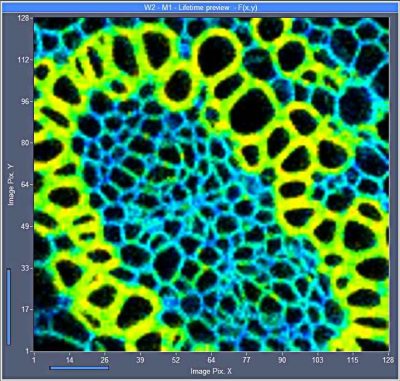
New SPCM Software Runs Online-FLIM at a Rate of 10 Images per Second

Small ps diode lasers deliver high power
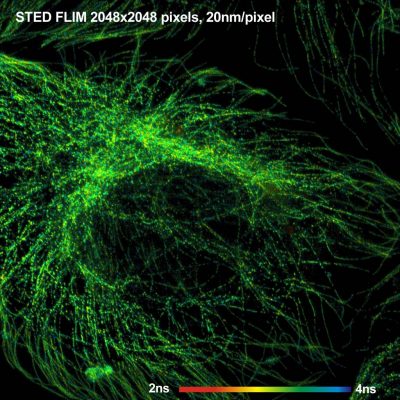
bh – Abberior Combination Records STED FLIM at Megapixel Resolution
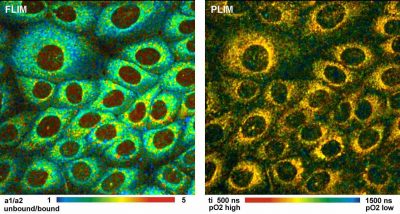
bh FLIM / PLIM technique simultaneously records pO2 and NAD(P)H unbound/bound ratio

PML-16 GaAsP multi-wavelength detector is 6 times more sensitive than predecessor with conventional cathode
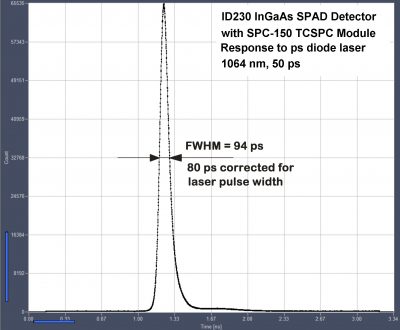
80 ps FHWM with ID230 InGaAs SPAD and SPC 150 TCSPC Module
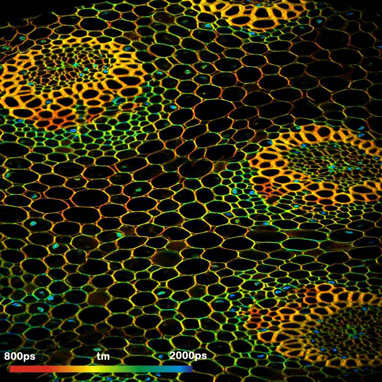
DCS-120 MP System Records Multiphoton FLIM and PLIM

bh TCSPC System Records FLIM with Piezo Stage
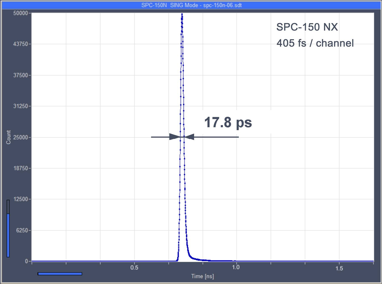
World Record in TCSPC Time Resolution: Combination of bh SPC-150NX with SCONTEL NbN Detector yields 17.8 ps FWHM
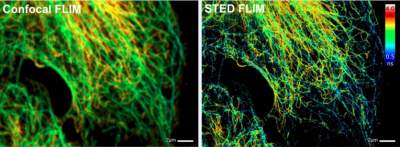
bh SPC-150 Modules Record Super-Resolution FLIM in Abberior STED FLIM Microscopes
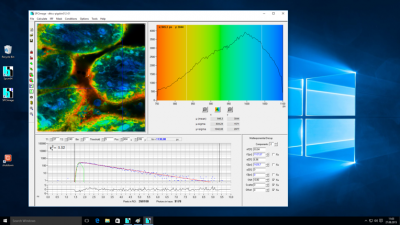
bh TCSPC software runs under Windows 10
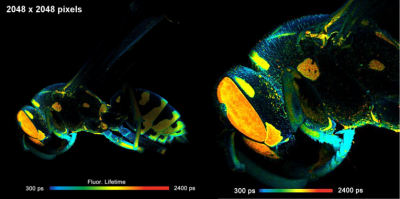
DCS-120 MACRO system records FLIM of cm-size objects
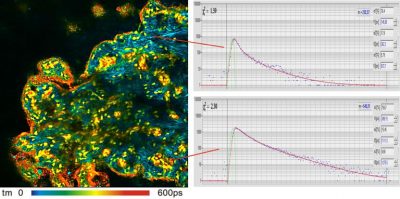
bh FLIM systems record FLIM and FCS with Zeiss BiG 2 detectors
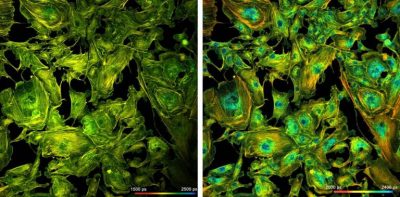
DCS-120 System Records FLIM at Megapixel Resolution
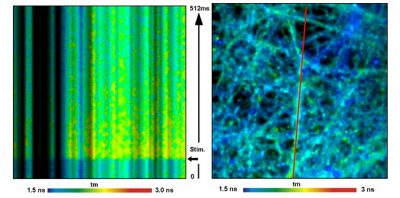
bh FLIM Systems Record Calcium Transients in Live Neurons

bh TCSPC Detects Mouse Behaviour

Megapixel FLIM – the New 64 bit SPCM Software
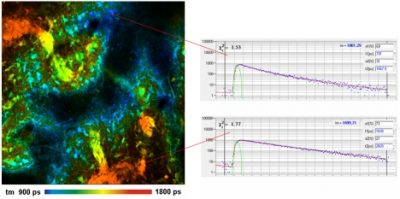
Multiphoton NIR FLIM with the Zeiss LSM 7MP OPO System

New BDL-SMN Picosecond Diode Lasers
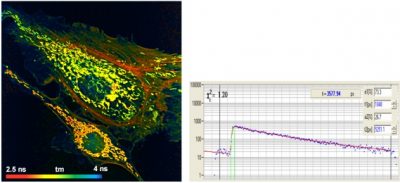
Multiphoton FLIM with the Leica HyD RLD Detectors