Advanced time-correlated single photon counting (TCSPC) and picosecond diode lasers were used to record light-induced transients of the fluorescence lifetime of chlorophyll in living plants. The experiment setup is almost free of optical components and essentially built up from bh picosecond diode lasers, photon detectors, and TCSPC devices. Both photochemical quenching and non-photochemical quenching transients are resolved, with a resolution of 100 μs per decay curve.
Recording the Kautsky Effect by Fluorescence Lifetime Detection
W. Becker, A. Bergmann, G. Biscotti, Becker & Hickl GmbH, Berlin
Abstract: Advanced time-correlated single photon counting (TCSPC) and picosecond diode lasers were used to record light-induced transients of the fluorescence lifetime of chlorophyll in living plants. The experiment setup is almost free of optical components and essentially built up from commercially available lasers, detectors, and TCSPC modules. Both photochemical quenching and non-photochemical quenching transients are resolved, with a resolution of 100 µs per decay curve.
Fluorescence Transients of Chlorophyll
When a plant or a living plant cell is exposed to light the intensity of the chlorophyll fluorescence shows characteristic changes. These changes were found by Kautsky and Hirsch in 1931 and have been termed fluorescence induction, fluorescence transients, or Kautsky effect [5, 6, 9]. The general behaviour of the fluorescence intensity is shown in Fig. 1.
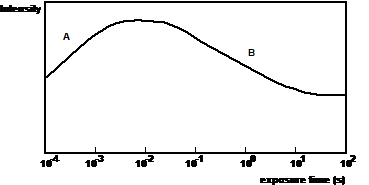
Fig. 1: General behaviour of the chlorophyll fluorescence after exposing a dark-adapted leaf to light. The fluorescence intensity first increases due to a decrease of photochemical quenching (a) and then decreases due to an increase of non-photochemical quenching (b).
When the light is switched on the fluorescence intensity increases with a time constant in the microsecond or millisecond range. After a few seconds the intensity falls again and finally reaches a steady-state level. The initial rise of the fluorescence intensity is attributed to the progressive saturation of the reaction centres in the photosynthesis pathway. Therefore the quenching of the fluorescence by the photosynthesis decreases with the time of illumination, with a corresponding increase of the fluorescence intensity. The quenching by the photosynthesis pathway is called photochemical quenching.
The slow decrease of the fluorescence intensity at later times is termed non-photochemical quenching. Non-photochemical quenching is most likely due to a protection mechanism the plant has to avoid photodamage. The processes that lead to non-photochemical quenching are often referred to as photoinhibition.
A large number of experimental setups to measure the chlorophyll transients are used [4, 6, 8]. Often a continuous light of variable intensity is applied simultaneously with a modulated light signal of constant intensity. By detecting only the fluorescence signal at the modulation frequency, the fluorescence efficiency as a function of intensity and time is recorded. A second technique uses an intense flash of light to close the reaction centres and records the fluorescence intensity before and after the flash. The problem in all these experiments is that any change in the fluorescence intensity can either be caused by a change in the rate of the fluorescence quenching or by a change in the concentration of fluorescing chlorophyll molecules.
Changes in the quenching rate can easily be separated from concentration changes by recording the fluorescence lifetimes. A change in the quenching intensity causes a corresponding change in the fluorescence lifetime, a change in the concentration of the fluorescent species does not.
Recording transient changes in the fluorescence lifetime on the time scale faster than a few seconds is often considered impossible. Indeed, classic TCSPC devices based on nuclear instrumentation modules [10, 11] were limited to very low count rates and relatively long acquisition times. Moreover, the devices could only acquire a single decay curve, which had to be read out before the next acquisition cycle could be started. However, advanced TCSPC devices cannot only be operated at count rates two orders of magnitude higher than classic devices but also have sequential recording modes which are directly applicable to the recording of chlorophyll transients [1, 2, 3].
Non-Photochemical Quenching
A simple setup for recording the non-photochemical quenching transients is shown in Fig. 2.
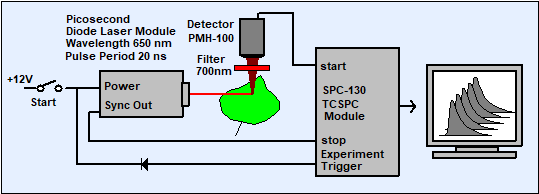
Fig. 2: Recording the non-photochemical quenching transient of chlorophyll a. The TCSPC module records a single sequence of fluorescence decay curves starting with the switch-on of the laser.
The fluorescence is excited by a picosecond diode laser. The fluorescence light emitted by the sample is separated from the scattered excitation light by a bandpass filter and detected by a PMT. The photon pulses from the PMT are used as start pulses, the reference pulses from the laser as stop pulses of the TCSPC module. When the laser is switched on a recording sequence in the TCSPC module is triggered. A trigger signal is obtained by connecting a diode from the operating voltage input of the laser to the TTL-compatible experiment trigger input of the TCSPC module.
For the results shown below a Becker & Hickl BHL-600 laser module was used. The wavelength was 650 nm, the pulse duration 80 ps, and the repetition rate 50 MHz . The incident power density in the excited spot of the leaf was approximately 1 mW/mm2. To keep the optical setup simple the fluorescence light was detected directly, i.e. without any relay lens, by a Becker & Hickl PMH‑100‑1 PMT module. To suppress scattered excitation light a 700 ± 15 nm bandpass filter was inserted into the PMH‑100‑1. The setup is further simplified by the fact that the PMT modules is powered directly from the TCSPC module, i.e. does not need any external high voltage power supply. The fluorescence decay curves were recorded in one TCSPC channel of a Becker & Hickl SPC-134 system. In principle, any other bh TCSPC module can be used as well. The TCSPC module was operated in the f(t,T) mode [2] to obtain one decay curve each 2 seconds. The count rate was approximately 106 s-1 so that about 2×106 photons are recorded per decay curve. Dead time compensation [2] was used to avoid the influence of counting loss on the recorded intensity. Typical results are shown in Fig. 3.



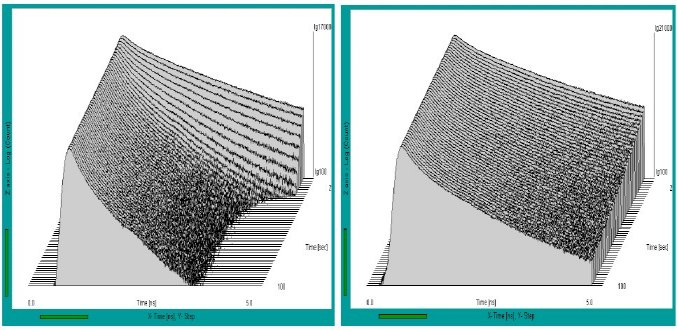
Fig. 3: Sequences of fluorescence decay curves of leaves after start of illumination. Left to right: Fresh leaf, faded leaf, dried leaf. Time per curve 2 seconds, logarithmic intensity scale. The sequence starts from the back.
For better visibility of the lifetime changes, the sequence starts at the back. Fig. 3, left, shows a sequence recorded at a fresh leaf. There is a considerably decrease in the fluorescence lifetime, but almost no change in the peak intensity of the decay curves. This shows that the recorded transient is almost essentially a change in the quenching rate.
The decay sequences show remarkable differences depending on the health state of the leaf. In a fresh leaf (left) the fluorescence lifetime decreases considerably in the few seconds of illumination. In a faded leaf (middle) the effect is slower and less pronounced. A dry leaf (right) does not show any noticeable lifetime changes.
Photochemical Quenching
The recording of the photochemical-quenching transient requires a time resolution of the order of 100 µs per curve. A sequence this fast cannot be reasonably recorded in a single-shot experiment. Therefore, the recording of the sequence is repeated and the data accumulated until enough photons have been collected. The used setup is shown in Fig. 4.
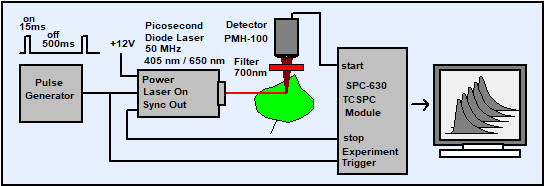
Fig. 4: Recording the photochemical quenching transient of chlorophyll. The laser is periodically switched on for 15 ms. A fast recording sequence is started at the beginning of each laser on interval, and a large number of recording cycles is accumulated.
The setup uses a picosecond diode laser with fast on/off control capability. For the results shown below both a BDL-475 laser (465 nm) and a BHLP-700 laser (650 nm) were used. The laser were periodically switched on and off by a pulse generator. The on duration is 15 ms, the period 515 ms. Within the on phases, the laser delivers picosecond pulses at its normal pulse period of 20 ns. The laser beam excites the fluorescence in the sample and, simultaneously, initiates a photochemical quenching transient. The fluorescence photons are detected by the PMH‑100‑1 PMT module and recorded in the TCSPC module. Each laser on transition triggers a fast hardware-controlled sequence of 128 decay curves with an acquisition time of 100 us per curve. For the results presented here an SPC-630 module was used and operated in the continuous flow mode [3]. Other bh TCSPC modules and other hardware-controlled sequential modes can be used as well [2].
The rate of photochemical quenching decreases with a time constant of about 2 ms within the 15 ms on period. Consequently, the fluorescence lifetime increases with the same time constant. In the subsequent off period the photochemical-quenching rate recovers to its initial state.
Of course, the number of photons recorded within one decay curve of a single on-off period is not sufficient for any reasonable lifetime analysis. Therefore, a large number of on-off cycles are accumulated. Due to the low duty cycle of the laser on signal, the average excitation intensity is low. It does therefore not induce much non-photochemical quenching. Typical results are shown in Fig. 5. 10,000 on/off cycles were accumulated. The sequence starts at the front.


Fig. 5: Photochemical quenching transient of chlorophyll. The sequence starts at the front. Time per curve 100 us, 10,000 on/off cycles accumulated. Left: Excitation at 650 nm. Right: Excitation at 465 nm, the double peak is caused by an afterpulse of the laser.
Although there is a clear change in the fluorescence lifetime no change in the peak intensity of the curves is visible. This shows that the photochemical quenching transient is indeed caused by a change in the quenching efficiency, not by a change in the concentration of fluorescing chlorophyll molecules.
Summary
The results show that advanced TCSPC modules can be used to record fluorescence lifetime transients both for photochemical and for non-photochemical quenching. The experimental setup is very simple, i.e. almost free of optical components and only built up from commonly available components. The transients were recorded with a time resolution down to 100 µs per decay curve.
References
1. Becker, W., Bergmann, A., Biscotti, G., Rück, A., Advanced time-correlated single photon counting technique for spectroscopy and imaging in biomedical systems. Proc. SPIE 5340, 104-112 (2004)
2. Becker & Hickl GmbH, SPC-134 through SPC-830 time-correlated single photon counting modules, application manual (2004). Available on www.becker-hickl.com
3. Becker, W., Hickl, H., Zander, C., Drexhage, K.H., Sauer, M., Siebert, S., Wolfrum, J., Time-resolved detection and identification of single analyte molecules in microcapillaries by time-correlated single photon counting. Rev. Sci. Instrum., 70, 1835-1841 (1999)
6. Govindjee, Seufferheld, M.J., Non-photochemical quenching of chlorophyll α fluorescence: Early history and characterization of two xanthophyll-cycle mutants of Chlamydomonas Reinhardtii. Funct. Plant Biol., 29, 1141-1155 (2002)
7. Kolber, Z.S., Práil, O., Falkowski, P.G., Measurements of variable chlorophyll fluorescence using fast repetition rate techniques: defining methodology and experimental protocols. Biochimica et Biophysica Acta, 1367, 88 (1998)
8. Kolber, Z.S., Laser Induced Fluorescence Transient (LIFT) Method for Measuring Photosynthetic Performance and Primary Productivity in Terrestrial Ecosystems. Earth Science Technology Conference, Paper B1P2, Pasadena, California (2002)
9. Maxwell, K., Johnson, G.N., Chlorophyll fluorescence - a practical guide. Journal of Experimental Botany 51, 659-668 (2000)