FLIM Systems Since 1998 – Fast and Reliable – Ultra-High Resolution – Entry Level to High-End
Proceed to Technical Descriptions:
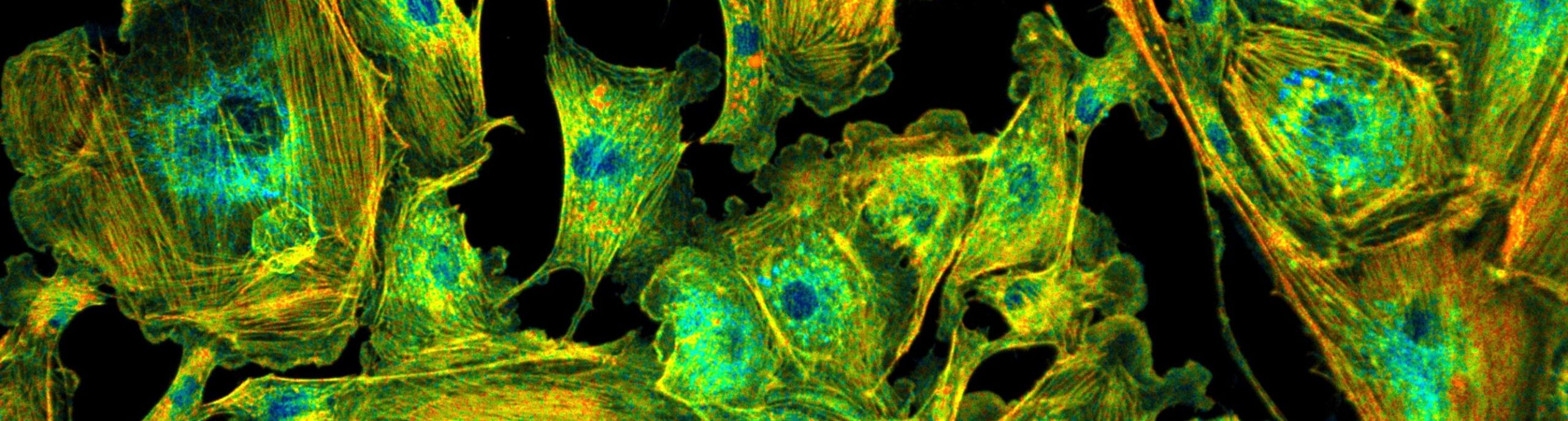
Why Use bh FLIM
About bh’s FLIM Technique
Bh’s FLIM technique uses a combination of single-photon detection and confocal or multiphoton laser scanning. By doing so, it combines the most accurate techniques of resolving optical data in space and in time. Time-correlated single-photon detection delivers fluorescence decay data at ideal sensitivity and unprecedented time resolution, and scanning delivers three-dimensional images that are free of lateral and longitudinal scattering and out-of-focus blur. By combining these features, bh FLIM outperforms any camera-based FLIM technique in image quality and time resolution.
FLIM is often considered just a contrast technique for improving the discrimination of different fluorophores in microscopy. However, this is by far not all. By delivering high-accuracy spatially resolved fluorescence decay data bh’s TCSPC FLIM technique makes an almost ideal Molecular Imaging technique. The fluorescence decay functions recorded in the pixels of a FLIM image bear information on the molecular environment of the fluorophores. With its single-photon sensitivity, live-cell compatibility, and optical sectioning capability, TCSPC FLIM is therefore an ideal imaging technique for live-science applications. It delivers information on protein interactions, interaction with drugs, metabolic state of cells and tissues, concentration of physiological relevant ions, pH and oxygen concentration, redox states, and other biologically relevant parameters.
From the beginning, bh FLIM systems (and bh FLIM data analysis!) have been designed with these applications in mind. Life-science applications are therefore supported with technical capabilities like multi-wavelength FLIM, Z-stack FLIM, time-series FLIM, recording of fast physiological effects, label-free imaging, excitation-wavelength multiplexing, metabolic FLIM, and simultaneous FLIM / PLIM, and data analysis functions like multi-exponential decay analysis, MLE, FRET analysis, Metabolic imaging, and GPU processing. Good reasons to use bh FLIM systems.
FLIM Systems from Entry Level to High-Performance Molecular Imaging
Based on their proprietary Multi-Dimensional TCSPC Technique, Becker & Hickl were first to introduce FLIM systems to the life science market. FLIM systems for ophthalmoscopy were introduced already in 1996 and FLIM systems for microscopy in 1998. Since then, bh FLIM systems have constantly been improved with new TCSPC devices, optical systems, scanners, light sources, and detectors. Novel functions have been added, and previously unknown applications in life sciences have been addressed, please see bh FLIM: More than Fluorescence-Lifetime Imaging for an 8-page summary. Now, Becker & Hickl are offering complete one-photon and multiphoton Microscopy FLIM systems including lasers, microscopes, scanning devices, detection optics, detectors, TCSPC electronics, and data acquisition and data analysis software, FLIM Systems for macroscopic samples, and one-photon and multiphoton FLIM systems for a wide range of laser scanning microscopes of external manufacturers. To facilitate applications of these systems Becker & Hickl provide an unprecedented amount of free product and application literature. Please see, for example
The bh TCSPC Handbook, 1032 pages, currently out in the 10th edition, 1032 pages
DCS-120 Confocal and Multiphoton FLIM Systems, user handbook, 452 pages
FLIM Systems for Zeiss LSM Family Laser Scanning Microscopes, 356 pages
FLIM Systems for Laser Scanning microscopes, overview brochure, 40 pages
DCS-120 confocal and multiphoton FLIM Systems, Overview Brochure, 48 pages
Bigger and Better Photons – The Roady to Great FLIM Results. Education brochure. 45 pages
For more information on Becker & Hickl FLIM systems and fluorescence lifetime imaging microscopy please check ‘Literature‘.
For further support and product inquiries, please use our contact form or call us at +49 (30) 212 80 02-0.
Ultra-High Resolution FLIM
bh FLIM systems reach lifetime resolution down to the sub-10 ps range. This is a factor of 5 to 10 more than FLIM systems of other manufacturers. Ultra-high time resolution does not only facilitate molecular imaging, FRET Imaging and metabolic imaging in general, it also opens a whole world of previously unknown FLIM applications. Please see, for instance
Ultra-Fast Fluorescence Decay in Malignant Melanoma
Ultra-Fast Fluorescence Decay in Natural Carotenoids
High-Resolution Multiphoton FLIM Reveals Ultra-Fast Fluorescence Decay in Human Hair
Two-Photon FLIM of Mushroom Spores Reveals Ultra-Fast Decay Component
Applications of Fluorescence Lifetime Imaging Microscopy
Becker & Hickl FLIM systems cover the full range of applications from simple contrast enhancement in microscopy to molecular imaging in life sciences. Frequent molecular-imaging applications are
- Measurement of ion concentrations
- pH measurement
- Lable-free imaging by FLIM of endogenous fluorophores
- Observation of differentiation of stem cells
- Metabolic Imaging by NADH and FAD FLIM
- Detection of tumors
- Investigation of cancer origins, cancer development and cancer progression
- Monitoring of drug action on the cell level
- FRET Imaging
- Diffusion of nanoparticles in skin
- Drug delivery
- Personalised Chemotherapy
- Simultaneous NAD(P)H and pO2 Imaging
- In vivo diagnostics of skin
- Ophthalmic imaging
Please see bh TCSPC Handbook, Chapter ‘Biological FLIM Applications’. In all these applications, high sensitivity, high photon effciency, high time resolution, and the ability to resolve complex fluorescence decay functions are key. Reasons enough to chose a Becker & Hickl FLIM system.
FLIM Data Analysis: Get Fit for Molecular Imaging
Data analysis is performed by bh’s legendary SPCImage NG software. SPCImage NG derives multi-exponential decay parameters from the data within individual pixels, groups of pixels, and ROIs. It does so with near-ideal signal-to-noise ratio, at minimum crosstalk between the decay components, minimum crosstalk between the decay parameters and the photon number, and within a processing time no longer than a few seconds. It converts these parameters into biologically relevant quantities, such as FRET distances or metabolic ratios, and diplays the results as meaningful and beautiful colour-coded images. SPCImage NG functions include
- Single, double, triple exponential decay models
- Incomplete-decay option to analyis slow decays
- Shifted component option for Ophthalmic FLIM
- MLE procedure for accurate fit a low photon number
- GPU processing to obtain results within seconds
- Combination with Phasor Plot
- Image segmentation via phasor plot
- FLIM Analysis of moving objects
- Automatic IRF generation – no need to measure IRFs
- Images of metabolic parameters in cells and tissues
- Images of FRET intensities and FRET distances
- FRET analysis without need of a reference lifetime
- Images of amplitudes and lifetimes of decay components
- Ratio images of amplitudes or lifetimes
- Ratio images calculated from images of two spectral channels
- Batch Processing for time series or Z stack data
For details, please see
- SPCImage Data Analysis
- TCSPC Handbook, chapter SPCImage NG data analysis Software
- SPCImage NG, overview brochure
For further support and product inquiries, please use our contact form or call us at +49 (30) 212 80 02-0.


