Multiphoton NDD FLIM at NIR Detection Wavelengths with the Zeiss
LSM 7MP and OPO Excitation
Wolfgang
Becker, Vladislav Shcheslavskiy, Becker & Hickl GmbH
Abstract: We demonstrate multiphoton NDD FLIM of tissue
samples stained with near-infrared dyes. For the experiments we used a Zeiss
LSM 7MP multiphoton microscope with a Coherent Chameleon OPO (optical
parametric oscillator) as an excitation source. The excitation wavelengths
range from 1000 nm to 1300 nm. The fluorescence was detected by an
HPM‑100-50 NIR hybrid detector attached to the NDD (non-descanned
detection) port of the microscope; the FLIM data were recorded by a standard bh
TCSPC FLIM system. We demonstrate the performance of the system for tissue
samples stained with Methylene Blue, Indocyanin Green (ICG), and 3,3-Diethylthiatricarbocyanine
(DTTCC). All three dyes could be efficiently excited at wavelengths from 1200
nm to 1300 nm. The dyes showed remarkable variability in their fluorescence
lifetimes. The lifetimes clearly depended on the tissue structures the dyes
were located in.
Keywords: FLIM, TCSPC, Multiphoton Excitation, OPO,
NIR Dyes, Methylene Blue, Indocyanin Green, Fluorescence Lifetime
FLIM with NIR Fluorophores
Fluorophores emitting in the near-infrared
region are used as markers in diffuse optical tomography and small-animal
imaging. For these applications, it is important to know how the fluorescence
lifetime depends on the binding target and on the local molecular environment
on the cell level. Moreover, lifetime variation due to variable local molecular
environment may be exploitable as a probe function [7, 8]. Another advantage of NIR probes is
that there is no contamination from autoflourescence. Moreover, light propagation
through biological tissue at infrared wavelengths is less impaired by
scattering and absorption than in the visible range. We have recently shown
that even one-photon excitation and confocal detection in the NIR yields
surprisingly clear images from tissue layers up to 50 µm deep [2, 3, 5]. It can
be expected that the penetration depth is further enhanced by two-photon
excitation and non-descanned detection.
For the experiments described here we used
a Zeiss LSM 7MP with OPO excitation [9] and a bh Simple Tau 150 TCSPC FLIM
system [1, 2, 4]. To obtain sensitivity
in a wavelength range up to 900 nm the HPM‑100-40 GaAsP hybrid detector
of the FLIM system was replaced with an HPM-100-50 GaAs hybrid detector. The
detector was attached to the NDD port of the microscope via a Zeiss T adapter [4].
To be able to detect fluorescence up to 900 nm we replaced the standard
two-photon beamsplitter in the beam path at the back of the objective lens with
a 980 nm dichroic mirror. The beamsplitter is not critical, an 80/20
wideband beamsplitter can be used as well. The standard 700 nm short pass
(laser blocking) filter in the T adapter was replaced with a 980 nm short
pass filter. The microscope objective lens was an x20/1.0 DIC VIS-IR
Plan-Apochromate for water immersion.
FLIM Images
Methylene Blue
Methylene Blue is a biomedically
interesting compound. It has anti-viral and anti-bacterial effects, and it has
been evaluated as a drug against malaria [12]. It has been applied to induce
apoptosis in cancer cells [13], and to treat psoriatic skin lesions by
photodynamic therapy [11]. The use of methylene blue to treat Alzheimer disease
has been under clinical trial [10].
Methylene Blue has an absorption band from
550 to 690 nm, with a maximum at 660 nm. Fluorescence is emitted from
650 nm to 750 nm, with a maximum at 680 nm. It can thus be
expected that two-photon excitation works well in the range from 1000 nm
to 1200 nm.
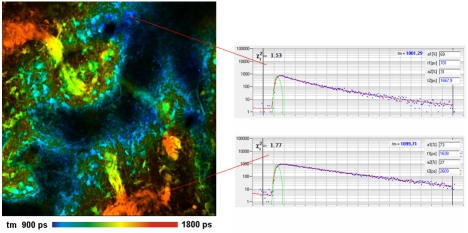
A FLIM image obtained from a pig skin
sample stained with Methylene Blue is shown in Fig. 1, left. The sample was
excited at 1200 nm, the fluorescence was detected from 680 nm to
780 nm. Excitation was surprisingly efficient. No more than 4 percent of
the available OPO power were needed to obtain a count rate on the order of
1 MHz. Decay curves from characteristic spots are shown in Fig. 1, right.
The decay profiles are multi-exponential but can be reasonably fitted by a
double-exponential decay model. The amplitude-weighted lifetime in different
parts of the tissue varies by almost a factor of two.
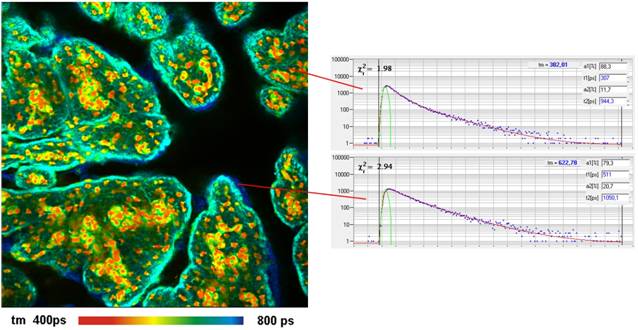
Fig. 1: Pig skin stained with methylene Blue. Left: Lifetime image,
double-exponential decay model, amplitude-weighted lifetime. Two-photon
excitation at 1200 nm, 512 x 512 pixels, 256 time channels. Right:
Decay curves in characteristic spots of the image.
Indocyanine Green (ICG)
FLIM Images of a similar sample stained
with ICG are shown in Fig. 2. The data were analysed by a double-exponential
decay model. The lifetime shown is the amplitude-weighted average of the decay
components. The fluorescence was excited at 1200 nm, fluorescence was
detected from 780 nm to 850 nm. As for the Methylene Blue, about 3 %
of the available excitation power was sufficient to obtain a count rate on the
order of 1 MHz.
The range of the lifetime and its
variability is in agreement with FLIM data obtained by one-photon excitation [3,
5]. The results show that the common assumption of an essentially invariable
fluorescence lifetime (and thus quantum efficiency) of ICG in biological
systems is not correct.
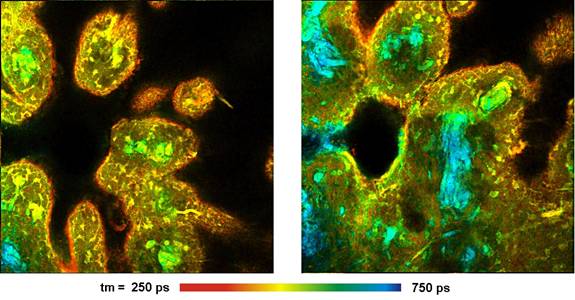
Fig. 2: Pig skin stained with Indocyanin
Green. Two-photon excitation at 1200 nm, detection from 780 to
850 nm. Amplitude-weighted lifetime of double-exponential decay. Depth
from top of tissue 10 µm (left) and 40 µm (right). 512x512 pixels,
256 time channels.
3,3-Diethylthiatricarbocyanine (DTTCC)
A FLIM image of a pig skin sample stained
with DTTCC is shown in Fig. 3. The excitation wavelength was 1200 nm, the
fluorescence was detected from 780 nm to 850 nm. The excitation power
was 2% of the available OPO power. The fluorescence lifetime is generally
longer than for ICG. The general lifetime behaviour is the same as found in [3,
5]. The lifetime is short where the tissue was exposed to high dye
concentration. However, there is also variability inside the tissue.
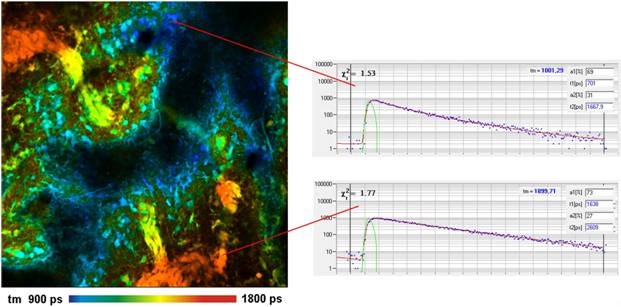
Fig. 3: Pig skin stained with DTTCC. Left: Lifetime image,
amplitude-weighted lifetime of double-exponential decay model, 512 x 512
pixels, 256 time channels. Right: Decay curves in selected spots of the image.
FLIM Z Stacks
To explore the dependence of the
fluorescence decay parameters on the staining conditions we recorded FLIM Z
stacks for the samples shown above. The Z stacks were recorded by the Mosaic
Imaging function of the SPCM 64-bit software [6]. Each Z stack contains 16 images from the
surface of the tissue down to about 60 µm. The results are shown in Fig. 4
through Fig. 6.
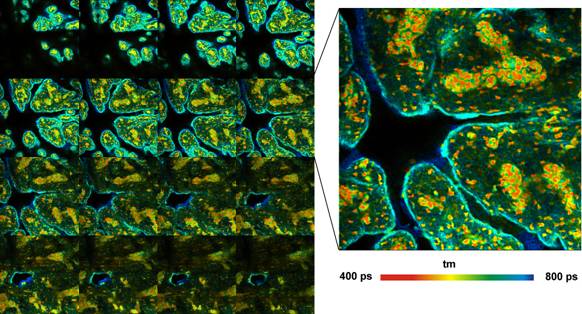
Fig. 4: Pig skin stained with Methylene Blue. Z-stack recorded by Mosaic
FLIM, 16 planes from 0 to 60 µm from top of sample, each plane 512x512
pixels, 256 time channels. Amplitude-weighted lifetime of double-exponential
decay. Plane 8 shown magnified on the right.
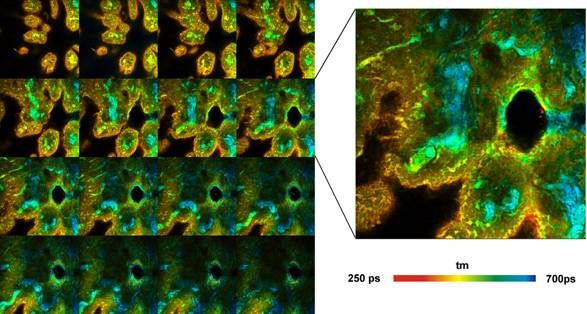
Fig. 5: Pig skin stained with Indocyanin
Green. Z-stack recorded by Mosaic FLIM, 16 planes from 0 to 60 µm from top
of sample, each plane 512x512 pixels, 256 time channels. Amplitude-weighted
lifetime of double-exponential decay. Plane 8 shown magnified on the right.
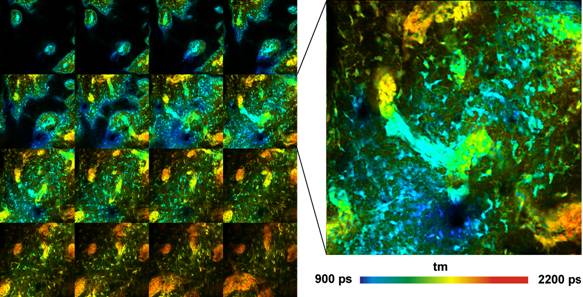
Fig. 6: Pig skin stained with DTTCC. FLIM Z-stack, recorded by Mosaic
FLIM, 16 planes, 0 to 60 um from top of tissue. Each element has 512x512 pixels
and 256 time channels per pixel. Amplitude-weighted lifetime of
double-exponential decay. Plane 8 shown magnified on the right.
The Z stacks show that, in the samples
investigated, the lifetime changes with the depth in the tissue. We used an
incubation time of only 15 minutes. It is therefore likely that the change is
caused by concentration changes or different binding efficiency due to
incomplete incubation. We did not explore the dependence on incubation time and
concentration. It appears, however, indicated to keep both under close control
to obtain reproducible lifetimes.
Summary
We presented a multiphoton NDD FLIM system
for deep-tissue lifetime imaging with near-infrared dyes. The system is based
an a Zeiss LSM 7MP laser scanning microscope with OPO excitation, and a bh
Simple-Tau 150 FLIM system with a HPM-100-50 near-infrared hybrid detector.
Methylene Blue, Indocyanin Green (ICG) and 3,3-Diethylthiatricarbocyanine
(DTTCC) could easily excited with the OPO system. No more than a few percent of
the available OPO power were needed. Lifetime images and FLIM Z stacks obtained
from stained pig skin showed remarkable resolution of the tissue structure and
surprisingly large variability in the fluorescence lifetime.
References
1.
Becker, W., Advanced time-correlated
single-photon counting techniques. Springer, Berlin, Heidelberg, New York, (2005)
2. Becker, W., The bh TCSPC handbook, 5th edition. Becker & Hickl
GmbH (2012), available on www.becker-hickl.com
3. W. Becker, V. Shcheslavskiy, Fluorescence Lifetime Imaging with
Near-Infrared Dyes. Proc. SPIE 8588 (2013)
4. Becker & Hickl GmbH, Modular FLIM systems for Zeiss
LSM 510 and LSM 710 family laser scanning microscopes, user handbook,
5th edition. Becker & Hickl GmbH (2012), available on www.becker-hickl.com
5.
Becker & Hickl GmbH, FLIM with NIR Dyes. Application note, available on www.becker-hickl.com
6. Becker & Hickl GmbH, Megapixel FLIM with bh TCSPC Modules -
The New SPCM 64-bit Software. Application note, available on
www.becker-hickl.com
7. M.Y. Berezin, H. Lee, W. Akers, S.
Achilefu, Near infrared dyes as lifetime
solvatochromic probes for micropolarity measurements of biological systems.
Biophys. J. 93, 2892-2899 (2007)
8.
M.Y. Berezin, W. J. Akers, K. Guo, G.M. Fischer,
E. Daltrozzo, A. Zumbusch, S. Achilefu, Long lifetime molecular probes based on
near-infrared pyrrolopyrrole cyanine fluorophores for in vivo imaging. Biophys.
J. 97, L22-L24 (2009)
9. Carl Zeiss Microscopy GmbH, New possibilities for multiphoton
microscopy from Carl Zeiss. OPO and simultaneous lasers for LSM 7 series.
Press release, www.zeiss.com
10. D. X. Medina, A.
Caccamo, S. Oddo, Methylene blue reduces Ab levels and rescues early
cognitive deficit by increasing proteasome activity. Brain Pathol. 21, 140149
(2011)
11. M. Salah, N. Samy, M. Fadel, Methylene blue mediated photodynamic
therapy for resistant plaque psoriasis. J. Drags Dermatol. 8, 42-49 (2009)
12. R. H. Schirmer, B. Coulibaly, A. Stich, M. Scheiwein, H. Merkle, J.
Eubel, K. Becker, H. Becher, O. Müller, T. Zich, W. Schiek, B. Kouyate,
Methylene blue as an antimalarial agent. Redox Report 8, 272-275 (2003)
13. G. T. Wondrak, NQQO1-activated phenothiazinium redox cyclers for the
targeted bioreductive induction of cancer cell apaptosis. Free Radic. Biol.
Med. 15, 178-190 (2007)