Precision Fluorescence-Lifetime Imaging of a Moving
Object
Wolfgang
Becker, Axel Bergmann, Becker & Hickl GmbH, Berlin, Germany
Abstract: We demonstrate precision lifetime
analysis on FLIM data of a moving object. The technique is based on
temporal-mosaic recording and image segmentation by the phasor plot of bh SPCImage
NG data analysis software. A cluster of phasors is selected in the phasor
space, identifying pixels of a given decay signature in the FLIM mosaic. These
pixels are back-annotated in the mosaic, selecting parts of the objects
irrespectively of their location in the individual images. The decay data of
the pixels within the selected areas are summed up. The result is a single
decay curve with extremely high pixel number which can be analysed at high
precision.
The Problem
The recording of fluorescence-lifetime
images of live objects is often impaired by motion in the sample. In cells
motion can be induced by moving mitochondria, in animals by heart beat or
simply by muscle activity. An example is shown in Fig. 1. It shows autofluorescence
images of the leg of a water flee. The insect is squeezed between the slide and
the cover slip. It moved the leg as it tries to escape. The images were
recorded by TCSPC FLIM [1] with a bh DCS-120 confocal FLIM system at
470 nm excitation wavelength [2]. The image format is 256 x 256
pixels, 256 time channels. The images were recorded in single frames of 0.5
seconds. Despite the fast scan time the images are impaired by motion artefacts.
The image on the right is even badly distorted as motion occurred during the
scan time.
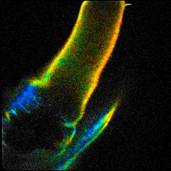
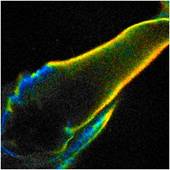
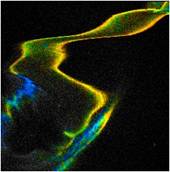
Fig. 1: Three images of a water flee, recorded with 0.5 s frame time.
Lifetime images, generated by SPCImage NG. MLE fit, double-exponential model,
mean (amplitude weighted) lifetime, binning 5 x 5 pixels.
The photon number in the individual pixels
ranges from about from about 3 to 30. Such low photon numbers are typical of
autofluorescence images. Even with binning of 5 x 5 pixels a
reasonable lifetime is obtained only in the bright pixels. It is therefore
desirable to increase the numbers of photons for the lifetime analysis.
However, accumulating the images over a longer period of time is no option
because motion would render the images useless. Higher count rate (by higher
excitation power) is not applicable as well because it would damage the object
under investigation within less than a minute. For the same reason, application
of a 'fast FLIM' technique is no solution. The limitation is in the photon rate
obtained from the sample, not in the counting capability of the FLIM system. Under
these conditions a 'fast' FLIM technique is no faster than 'normal' TCSPC FLIM [3].
The Solution: Temporal Mosaic FLIM
Mosaic FLIM was originally introduced in
the bh FLIM systems to record FLIM of large sample areas by sample stepping [1].
When used without sample stepping, the technique simply records a mosaic of
time-series images [1]. An example for the water flee is shown in Fig. 2.
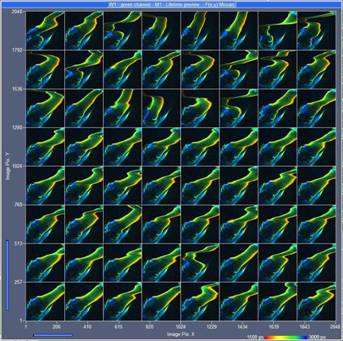
Fig. 2: Temporal Mosaic FLIM of a water flee. 64 images, each image recorded
in a single frame of 0.5 seconds. Images 256 x 256 pixels, 256 time
channels, lifetime display of bh SPCM data acquisition software.
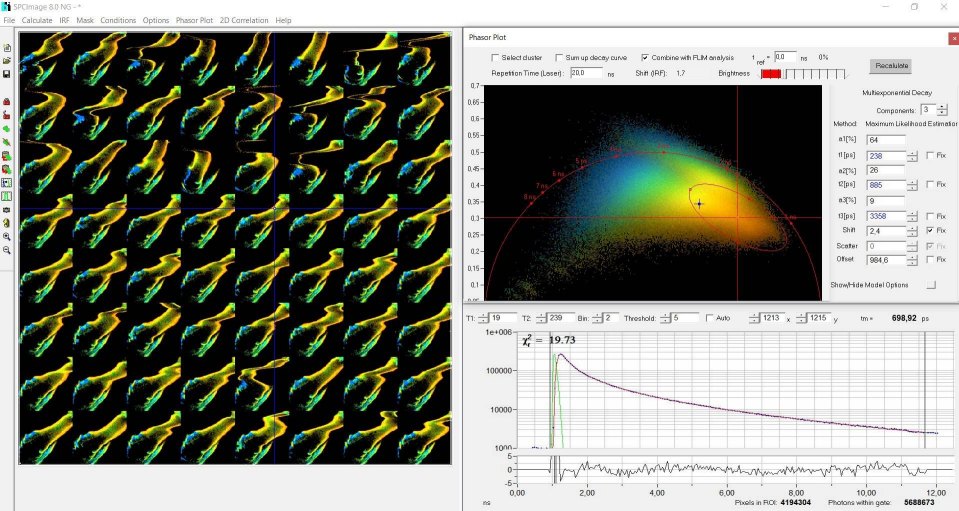
Data Processing by Phasor Image Segmentation
As expected, the individual images of the
mosaic are no better than the ones shown in Fig. 1. However, there is a
difference to a conventional time series. The FLIM mosaic is not a sequence of
individual FLIM data sets but a single photon distribution. It can therefore be
loaded into SPCImage data analysis software like a single image and analysed
the usual way [1, 5], see Fig. 3. It is then possible to calculate a phasor
plot over the entire mosaic. The result is shown in Fig. 4. The phasor plot
shows the pixels of the image (in this case the mosaic) in an amplitude-phase
diagram (the phasor space). The position in this diagram depends on the
temporal shape of the decay data, not on the position in the image. A cluster
of pixels selected in the phasor plot (ellipse in Fig. 4) therefore contains
pixels of similar decay signature - irrespectively of their location in the
image [1, 2].
Because the location in the phasor space
does not depend on the location in the image it also does not depend on
possible motion between the individual mosaic elements. The selection made in Fig.
4 selects pixels appearing orange in the FLIM image. Back annotation of the
selected pixels in the mosaic therefore selects the leg of the water flee, see Fig.
5. A combination of the decay data of the selected pixels in a single decay curve
is shown in Fig. 5, lower right. This curve contains more than 500 million
photons. It can thus be analysed at high precision. The decay parameters
obtained from a triple-exponential fit are shown in Fig. 5, upper right. A
similar result for the blue pixels of the image is shown in Fig. 6.
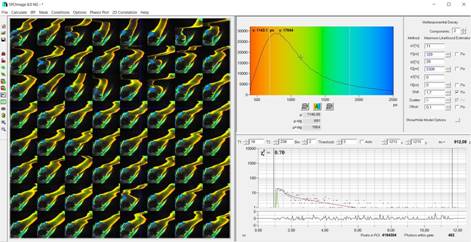
Fig. 3:
Temporal-FLIM Mosaic data loaded into SPCImage NG.
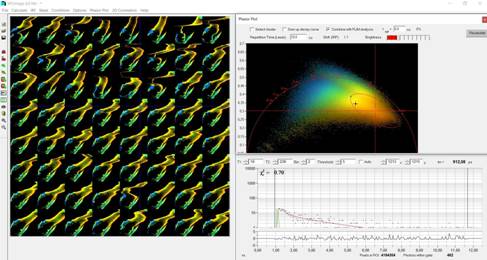
Fig. 4:
Temporal-FLIM Mosaic loaded into SPCImage NG, Phasor Plot activated
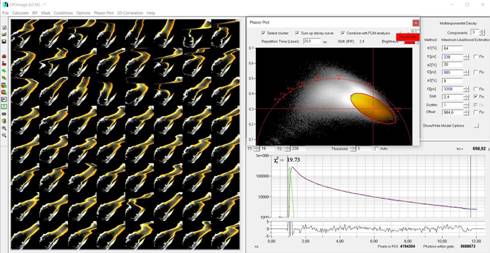
Fig. 5: Selection of orange pixels,
back-annotation in the mosaic, and combination into a single decay curve
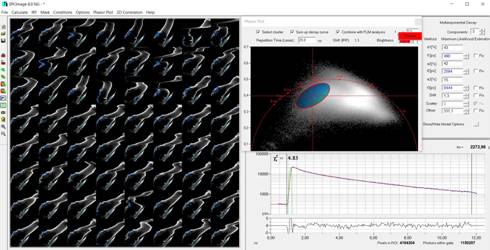
Fig. 6: Selection of blue pixels, back-annotation in the mosaic, and
combination into a single decay curve
Summary
Precision lifetime analysis on a moving
object is possible by recording a temporal mosaic of single frames, and
selecting clusters of a given decay signature in the phasor plot of SPCImage NG.
Pixels within the selected cluster represent parts of the object irrespectively
of their location in the individual elements of the FLIM mosaic. The decay data
of these pixels are summed up. The result is a single decay curve of high
photon number, which can be analysed at high precision.
References
1. W. Becker, The bh TCSPC handbook. Becker & Hickl GmbH, 9th ed.
(2021). Available on www.becker-hickl.com. Please contact bh for printed copies.
2.
Becker & Hickl GmbH, DCS-120 Confocal and
Multiphoton FLIM Systems, user handbook, 9th ed. (2021). Available on
www.becker-hickl.com
3.
Fast-Acquisition TCSPC FLIM: What are the
Options? Application note, available from www.becker-hickl.com
4. Becker & Hickl GmbH, SPCImage next generation FLIM data
analysis software. Overview brochure, available on
www.becker-hickl.com
5.
Becker & Hickl GmbH, New SPCImage Version
Combines Time-Domain Analysis with Phasor Plot. Application note, available on
www.becker-hickl.com
Contact:
Wolfgang Becker
Becker & Hickl GmbH
Berlin, Germany
Nunsdorfer Ring 6-9
https//www.becker-hickl.com
Email: becker@becker-hickl.com