New SPCImage Version Combines Time-Domain Analysis
with Phasor Plot
Wolfgang Becker, Axel Bergmann, Becker & Hickl
GmbH, Berlin, Germany
Abstract: Version 6.0 SPCImage FLIM
analysis software combines time-domain multi-exponential decay analysis with
the phasor plot. In the phasor plot, the decay data in the individual pixels
are expressed as phase and amplitude values in a polar diagram. Independently
of their location in the image, pixels with similar decay signature form
clusters in the phasor plot. Different phasor clusters can be selected, and the
corresponding pixels back-annotated in the time-domain FLIM images. The decay
functions of the pixels within the selected phasor range can be combined into a
single decay curve of high photon number. This curve can be analysed at high
accuracy, revealing decay components that are not visible by normal pixel-by
pixel analysis.
The Phasor Plot
TCSPC-FLIM data can be analysed both in the
time-domain and in the frequency domain. Time-domain analysis fits the decay
data in the individual pixels with a single or multi-exponential decay model,
or calculates lifetimes via the first moment of the decay functions [1].
Frequency-domain analysis transforms the decay data into the frequency-domain,
and expresses the decay data as amplitude and phase values at subsequent
harmonics of the repetition frequency. As it turns out, a good representation
of the decay signature is obtained already if only the phase and the amplitude
at the fundamental repetition frequency is used. Such data describe the
fluorescence decay in each pixel by just two numbers - the phase and the
amplitude of the first Fourier component.
Phase / amplitude data can be displayed and
analysed elegantly by the Phasor approach developed by the group of Enrico
Gratton. For each pixel, a pointer (the phasor) is defined and displayed in a
polar plot. The phase is used as the angle of the pointer, the modulation
degree as the amplitude [2, 3]. This phasor plot has several remarkable
features:
- The phasors of pixels with
single-exponential decay profiles are located a semicircle. The location on the
semicircle depends on the fluorescence lifetime
- Phasors of combinations of several decay
components are linear combinations of the phasors of the components
- Phasors of pixels with multi-exponential
decay profiles, i.e. sums of several decay components, end inside the
semicircle
- Pixels with similar amplitude-phase
values form clusters in the phasor plot. Pixels with similar decay signature
can thus be identified in the phasor plot, and combined for further analysis or
back-annotated in the image
Phasor analysis
does not explicitly aim on determining fluorescence lifetimes or decay components
for the pixels. Instead, it uses the phase and the modulation degree directly
to separate or identify fluorophores or fluorophore fractions, and determine
concentration ratios of different fluorophore fractions. The principle of the
phasor approach is shown in Fig. 1.





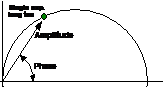



Fig. 1:
Relations between decay functions in the time domain (left) and phasors in the
frequency domain (right)
Implementation in SPCImage
Fig. 2 shows the main panel of the SPCImage
data analysis [1]. Data have been loaded, and analysed with a
double-exponential decay model. The panel shows an intensity image, a lifetime
image of the amplitude-weighted lifetime, a lifetime histogram over the pixels,
the decay curve at a selected position, and the corresponding decay parameters.
The images in Fig. 2 show cells expressing two fluorescent proteins. In two of
the cells (the green and orange one) the proteins are interacting, and FRET
occurs. The result is a double-exponential decay with a slow and a fast component
from the non-interacting and interacting donor, and, consequently, decrease in
the fluorescence lifetime [1]. The lifetime differences are clearly seen in the
lifetime image. The lifetime histogram shows three distinct peaks for the
different cells.
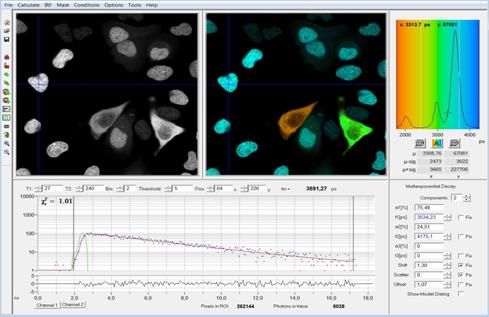
Fig. 2: SPCImage main panel. Intensity image, lifetime image, lifetime
histogram, decay curve at selected position, and decay parameters at selected
position. Recorded by bh Simple-Tau 152 FLIM system with Zeiss LSM 880.
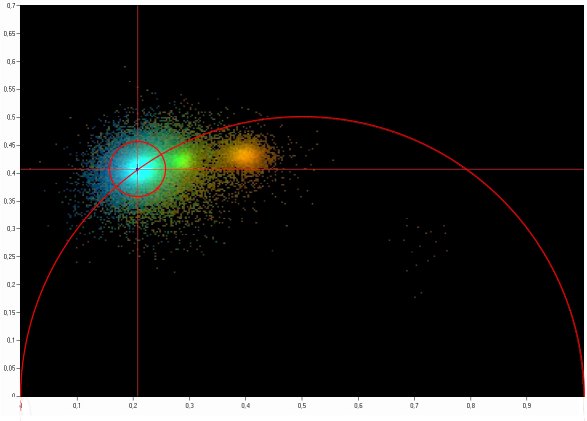
A click into Tools, Phasor Plot opens
the phasor plot panel. The relations between the lifetime image and the phasor
plot are shown in Fig. 3. Every pixel in the FLIM image (left) is represented
by a dot in the phasor plot (right). Pixels inside areas with different decay
profiles in the lifetime image are thus represented as different clusters of
phasors in the phasor plot. To make the correspondence between the image and
the phasor plot clearly visible SPCImage can assign the colours of the FLIM
image to the dots in the phasor plot. This function is activated by activating
the Combine with FLIM analysis option in the phasor plot. Cells of different
lifetimes therefore form separate clusters of phasors marked with different
colours in the phasor plot, see Fig. 3, right.
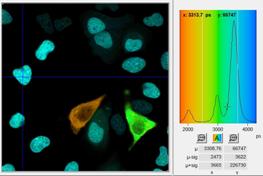
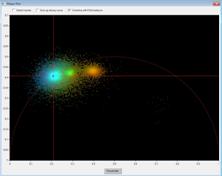
Fig. 3: Left: Lifetime image and lifetime histogram. Right: Phasor plot.
The clusters in the phasor plot represent pixels of different lifetime in the
lifetime image. Recorded by bh Simple-Tau 152 FLIM system with Zeiss LSM 880.
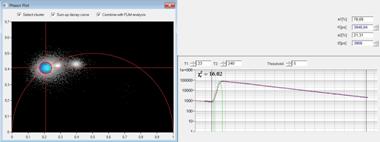
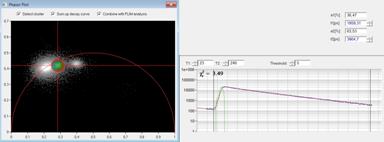
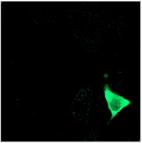
A cluster of interest in the phasor plot
can be selected by a cursor. By activating select cluster only pixel areas
with phasors inside the selected cluster area are displayed in the lifetime
images, see Fig. 4, right. Sum up decay curves in this situation combines the
decay data of all pixels inside the selected cluster area in a single decay
curve, see Fig. 4, middle. The result is a curve with an extremely high photon
number, as it is normally obtained only in a cuvette measurement. This curve
can be analysed at very high accuracy. In the case shown in Fig. 4 the combined
data show clearly that the blue cells have no interaction of the donor with an
acceptor. The decay function is a single exponential from the donor, with the
cluster on the semicircle in the phasor plot and two identical lifetime
components in the time-domain fit. The green cell has weak FRET, with an
interacting donor fraction of 1.95 ns lifetime. The yellow cell has strong
FRET, with an interacting donor fraction of 708 ps.
(Figure
continued page 4)
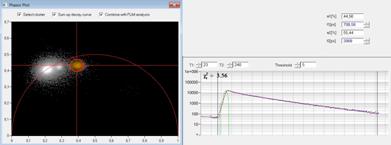
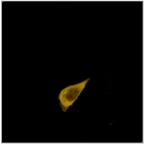
Fig. 4: Left: Selecting a cluster of phasors in the phasor plot. Middle:
Combination of the decay data of the corresponding pixels in a single decay
curve. Right: Display of the pixels corresponding to the selected cluster in
the phasor plot. Top to Bottom: Selection of different phasor clusters selects
cells with different decay signature.
Discussion
The combination of the phasor plot with
time domain fluorescence decay analysis identifies pixels of similar decay
signature in the frequency domain and back-annotates the corresponding image
areas in the time-domain FLIM images. This provides an intrinsic image
segmentation function. Separate images of different cells, different anatomic
features with characteristic decay signature or images for selected fluorophores
or fluorophore fractions can be obtained. The decay data of the pixels within
these areas can be combined in a single curve with substantially increased
photon number. This curve can be analysed at an accuracy comparable to that of
single-point decay measurements in cuvettes. Low-amplitude decay components or
decay components with almost similar lifetimes can thus be identified in the
data. It should be noted that pixels of similar signature can also be selected
in multi-parameter histograms in the time domain. These histograms either use several
independent parameters of multi-exponential decay functions [1] or fluorescence
decay times and intensity ratios in different wavelength intervals [4]. Also
these approaches deliver clusters of pixels with similar decay signature. The
advantage of the phasor plot is that the clusters are usually more clearly
defined than in decay parameter histograms, or do not depend on amplitude
ratios which may vary with filters, detectors, and absorption in the sample. It
should be noted, however, that all the cluster approaches are not always unambiguous.
Similar phasors or similar intensity ratios are not necessarily meaning that the
decay profiles of the corresponding pixels are similar. It is an advantage of
the method presented here that the combined decay profiles can be analysed at
high precision, and the source of unexpected decay components be identified.
Acknowledgements
The data used in this application note were
recorded on the EMBO Workshop on Biomolecular Interaction Analysis 2016: From
Molecules to Cells, in Porto, Portugal, 2019. We thank Kees Jalink of NKI
Amsterdam and Ana Seixas and Maria Azevedo, IBMC Porto for donating the cell material
and preparing the samples. Moreover, we thank Uros Krzic of Zeiss for donating
an LSM 880 to the FLIM experiments, and Valeria Caiolfa and Moreno Zamai for
organising the tutorial session.
References
1.
W. Becker, The bh TCSPC Handbook, 6th edition.
Becker & Hickl GmbH (2015). Available on www.becker-hickl.com, printed
copies available from bh
2.
Digman, M.A., Caiolfa, V R., Zamai, M. &
Gratton, E. The phasor approach to fluorescence lifetime imaging analysis.
Biophysical J. 94, L14-L16 (2008)
3.
M.A. Digman, E. Gratton, The phasor approach to
fluorescence lifetime imaging: Exploiting phasor linear properties. In:
L.Marcu, P.W.M. French, D.S. Elson, Fluorescence lifetime spectroscopy and
imaging. CRC Press, Taylor & Francis Group, Boca Raton (2015)
4.
S. Weidkamp-Peters, S. Felekyan, A. Bleckmann, R. Simon, W.
Becker, R. Kühnemuth, C.A.M. Seidel. Multiparameter
fluorescence image spectroscopy to study molecular interactions. Photochem.
Photobiol. Sci. 8, 470-480 (2009)