Shifted-Component Model Improves FLIO Data Analysis
W. Becker, A. Bergmann, Becker & Hickl GmbH, Berlin,
Germany
L.
Sauer, University of Utah, Salt Lake City, USA
Abstract: We present a new model for analysis of
fluorescence-lifetime ophtalmoscopy (FLIO) data. The model uses three
exponential components, two of which describe the fundus fluorescence, whereas
the third one models the fluorescence of the crystalline lens. The third
component is shifted toward short times, accounting for the difference in signal
transit time. Compared with the standard triple-exponential model, the fit
stability and the lifetime reproducibility are massively improved. Most
importantly, the new model allows us to separate the decay components from the
fundus from the decay component of the lens. We demonstrate the performance of
the new model on FLIO data of a cataract patient who obtained a cataract
surgery. Pre-surgery data were dominated by lens fluorescence. Analysed with
the conventional three-component model, the data did not deliver useful
information about the fundus. With the new model we were able to extract fundus
lifetimes which matched the lifetimes from post-surgery images.
Fluorescence-Lifetime Imaging Ophthalmoscopy
TCSPC FLIM is so sensitive that it can be
used to record fluorescence-lifetime images of the human retina in vivo.
Fluorescence-Lifetime Imaging Ophthalmoscopy, or FLIO, is sensitive to the
metabolic state of the tissue. It thus bears the potential to detect early
changes in the metabolism of the retina before these have caused irreversible
damage. The technique is in use since 1996, and has resulted in an impressive
number of research papers [1-35]. FLIO obtained a new push with the
introduction of the Heidelberg Engineering FLIO eye scanner, containing bh
TCSPC FLIM modules, bh ps diode lasers, and bh HPM hybrid detectors [33, 37]. Fluorescence
decay times of the fundus structures range from 200 to 600 ps, with component
lifetimes down to less than 80 ps. FLIO data analysis has therefore always been
tricky, requiring user interaction and experience to set the fit parameters
appropriately. Nevertheless, absolute fluorescence lifetimes obtained by
different instruments and different users differed noticeably. This application
note analyses the sources of the problem, and presents a new approach which
considerably improves the reproducibility of the results.
The Challenges of FLIO Data Analysis
1. The Instrument Response Function (IRF) is not exactly known
The Heidelberg Engineering FLIO
(fluorecence-lifetime ophthalmoscope) system records lifetime images of the
fundus of the human eye [37]. The decay data contain fluorescence decay
components down to less than 100 ps [25]. Under these circumstances, the
recorded decay functions are a convolution of the true fluorescence decay
functions with the temporal instrument response function (IRF). This is the
waveform the system would record for an infinitely short fluorescence decay
time [37].
The convolution operation cannot be
analytically reversed, i.e. a straightforward de-convolution procedure does not
exist. The task is solved by an iterative fit procedure:
-
Convolute the model function, 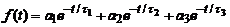 , with the IRF
, with the IRF
- Compare result with measured fluorescence
data
- Change the model parameters until best
fit is obtained
- Repeat the procedure for all pixels of
the FLIO data set
Obviously, the IRF has to be known to run
this procedure. It is, however difficult, if not impossible, to measure the IRF
accurately in a FLIO instrument. A fluorophore with a sufficiently short
lifetime (<5ps) and sufficient fluorescence quantum efficiency does not
exist. Using a simple scattering target instead faces the problem that the
detection system optically blocks the excitation wavelength. To detect the
scattered signal modifications have to be made to the optical system, which, in
turn, have an influence on the IRF. Moreover, multiple scattering in the target
broadens the signal. As a result, the IRF is, if detected at all, recorded
broader than it actually is.
2. The optical path length between the scanner and the
fundus varies
In FLIO measurements the optical path
length from the scanner to the fundus and back is not constant. It depends on
the patient's head profile and on the optical length of the eye. Differences of
1 cm in the distance (or 2 cm in the path length) are not unusual.
This translates in a transit-time difference of 67 ps. The uncertainty in
the transit time results in an uncertainty of the same size in the recorded
lifetime [37]. That means lifetimes on the order of 100 ps cannot be
determined without shifting the IRF into the correct temporal position.
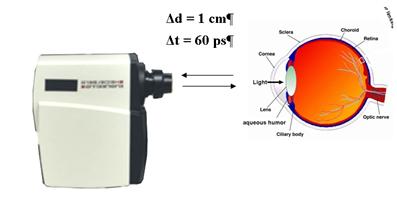
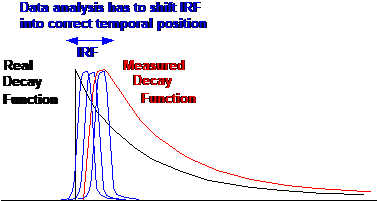
Fig. 1: The path length between the scanner
and the eye varies (left). The data analysis has to correct for the associated
transit time variation (right).
3. The commonly used model function does not describe the
decay profile correctly
The commonly-used model function is a
triple-exponential decay of the form
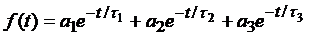 (1)
(1)
This model ignores the fact that the
fluorescence from the fundus is overlaid by fluorescence from the crystalline
lens. The lens fluorescence arrives 120 to 150 ps before the fundus
fluorescence. It not only adds an unwanted decay component to the net decay
function in every pixel, it also causes a distortion in the rising edge of the
fluorescence pulse (Fig. 2, left). The triple-exponential model (1) is not able
to describe the rising edge correctly (Fig. 2, right).
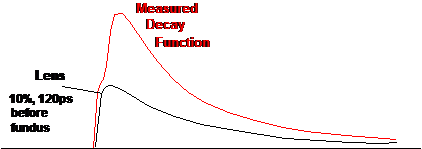
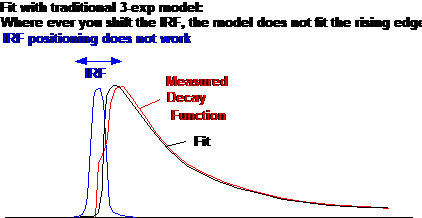
Fig. 2: Left: measured decay function in the presence of lens
fluorescence. Right: The model function is not able to describe the rising edge
correctly.
The inadequate modelling of the decay funtions
in combination with the inaccurately known IRF has consequences:
- The poor fit of the rising edge results in a broad χ2
minimum, ambiguity of the fit, and fit instability.
- There is no clear χ2 minimum for different IRF
positions. The fit routine is thus unable to find the correct IRF position.
Unstable IRF position leads to unstable lifetime results. Moreover, the
optimisation procedure tries to compensate for the distortion in the rising
edge by modifying the IRF position. Thus, even if an IRF position with minimum
χ2 is found it is not the correct one.
- The shape of the rising edge depends on the relative amount of
lens fluorescence detected. The shape has an influence on the IRF position
obtained, and thus on the lifetime determination. As a result, the fundus
lifetimes obtained from the fit depend on the focusing, and on the quality of
the patient's eye lens, in particular on the amount of astigmatism, and,
importantly, on the fluorescence properties of the lens. In particular, there
is a problem for cataract patients. These have extraordinarily high lens
fluorescence.
It has been attempted to solve these
problems by excluding the rising edge from the fit, i.e. by fitting only the
part of the decay functions after the maximum. This way, a good χ2 is
obtained for the falling part of the decay functions. However, the results
depend on the selection of the fit range and still contain an uncertainty from
the uncertainty of the IRF position. Moreover, fast decay components are not obtained
with the best possible accuracy. Information on these components is contained
mainly in the early part of the decay functions, i.e. in the part which is
excluded from the fit.
The Solution to Accurate FLIO Analysis
1. The Shifted-Component Model
The model function is extended with a
parameter, td3, which describes a shift of one of the fluorescence components.
The new model function is:
f(t) = a1 e-t/t1 + a2 e-t/t2 + a3 e(-t+td3)/t3
a1, a2, a3, t1, t2, t3 are fit parameters. td3
is the transit time from the lens of the eye to the fundus and back. A model
like this has already been suggested by D. Schweitzer [33]. Implementing the
model in FLIO data analysis was not successful, however, possibly because the delay was used as a fit parameter. This caused instability of the fit. We therefore assume
that td3 is constant. In reality, it may vary slightly with the
length of the eye. Our tests have shown, however, that the value of td3
is not critical. A td3 of 120ps to 150ps works well for all adults.
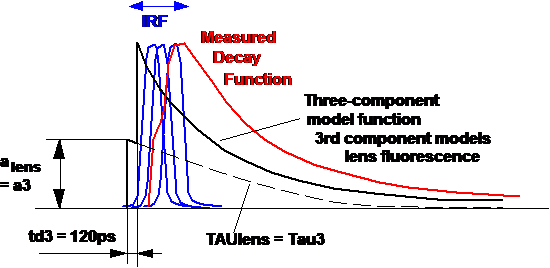
Fig. 3:
Modelling the eye fluorescence with the shifted-component model.
The fit delivers two decay components, t1
and t2, from the fundus, and a third component, t3, from
the lens. Thus, the shifted component model not only delivers an accurate fit
of FLIO data with a correctly positioned IRF, it also allows us to remove the contamination
of the fundus data by the lens decay component. This is achieved by calculating
an average (amplitude weighted) lifetime, tm12, only from the first
two decay components, t1 and t2 and their amplitude
factors a1 and a2:
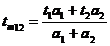 (2)
(2)
The calculation of tm12 has been
added to SPCImage, see sub-menu 'Colour', 'Encoding of'.
2. Synthetic IRF
We have to accept the fact that it is virtually
impossible to measure an accurate IRF for a FLIO system. We therefore defined a
mathematical model for the IRF. The model is composed of a term
f(t) = t/t0·e-t/t0 describing
the temporal response of the detector,
convoluted with
f(t) = e-(t2/tl)2 describing the
shape of laser pulse.
t0 characterises to detector, tl
the width of the laser pulse. t0 and tl are
characteristic to each FLIO instrument. They are one-time determined and stored
in the instrument software. (An IRF optimisation function is provided in
SPCImage but should be used by experts only)
Test of the Shifted-Component Model with a Fast Detector
To test our approach we recorded FLIO data
with an HPM-100-06 ultra-fast hybrid detector. The detector itself has an IRF
of less than 20 ps full width at half maximum [38], compared to about
120 ps for the HPM-100-40 standard detector [37]. Together with the laser
pulse the IRF width is on the order of 40 to 50 ps. With the fast IRF the
fit quality can be assessed more accurately than with the standard detector
(IRF width about 120 ps). The fact that the fast detector is less
sensitive [37] is insignificant for the test. The result is shown in Fig. 4.
The step in the rising edge of the
fluorescence (caused by lens fluorescence, compare Fig. 2 and Fig. 3) can
easily be seen. It stands out much more prominently than with the standard
detectors, where it forms only a kink in the edge. As can be seen in Fig. 4,
the model fits the step accurately. The χ2 distribution (shown below the
decay curve) is virtually free of bumps in place of the rising edge of the
fluorescence pulse.
It should be noted that fitting the data
did not require any tweaking of fit conditions or fit-interval boarders. The fit
interval was just set to the beginning and the end of the recorded decay data.
Small changes in the cursor positions had no visible influence on the results
as long as the left cursor remained left of the rising edge and the right
cursor in the region where the fluorescence has decayed to reasonably low
intensities.
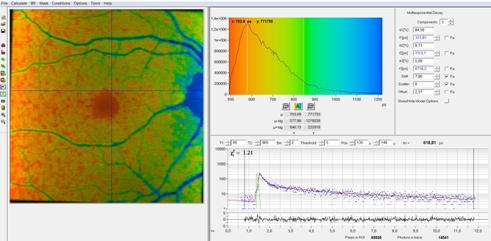
Fig. 4: Test
data recorded with ultra-fast hybrid detector. Analysis with shifted component
model and syntethic IRF.
Application to FLIO Data of a Cataract Patient
We applied the new analysis approach to
data from a cataract patient. The patient obtained a cataract surgery, i.e. got
an artificial lens implanted. The natural lens of a cataract eye is highly
fluorescent, whereas the artifical one is not. FLIO data were therefore
recorded before and after the surgery. The results are shown in Fig. 5 through Fig.
14. All images are shown in normal FLIM style, i.e. with the fluorescence intensity
as brightness and the fluorescence lifetime as colour. Opthlamology style is
different in that only the lifetime information is shown. We used the normal
FLIM style to give an impression of the contrast improvement obtained by the
new model. Instructions for SPCImage parameter setup are available in [39].
Fig. 5 is a pre-surgery image from the
short-wavelength channel with the normal tm, the amplitude-weighted
lifetime of a triple-exponential model. As can be seen from the figure, the
lifetime is entirely out of the normal interval of FLO data. The reason is the
strong contribution from long-lifetime lens fluorescence.
Fig. 6 is a tm12 image from the
same data set. tm12 contains only t1 and t2,
i.e. decay components from the fundus. tm12 is in the range of
normal FLIM data. More interestingly, tm12 is very close to the tm
of the post-surgery image, Fig. 11. The post-surgery data contain less or no fluorescence
from the lens. The similarity of the lifetimes indicates that tm12
of the pre-surgery image is really associated with the fundus.
Of course, the large contribution of lens
fluorescence has also an effect on the pixel intensities. The lens fluorescence
is spatially unspecific, and thus causes a decrease in image contrast. The
contrast can be improved by using time-gated pixel intensities from the early
part of the decay. This part is dominated by the fundus fluorescence and thus delivers higher contrast. The result is shown in Fig. 7.
A similar, yet less pronounced tendency is
found in the pre-surgery images of channel 2 (long wavelength channel). In this
wavelength channel the lens fluorescence is weaker than in Channel 1. Nevertheless, the tm12 image (Fig. 9) shows generally shorter lifetimes than the
tm image (Fig. 8). The tm12 lifetimes are close to the post-surgery tm
lifetimes, see Fig. 13. The gated-intensity image, Fig. 10, has increased
contrast, although the improvement is smaller than in channel 1.
Fig. 11 through Fig. 14 are the
post-surgery images. Importantly, the tm lifetimes in the
post-surgery images closely match the fundus lifetimes, tm12, in the corresponding
pre-surgery images. The tm12 image in channel 1 of the post-surgery
data shows a decay component, t3, that is clearly associated to the
front part of the eye. This is indicated by the kink in rising edge and the perfect
it of it. The lifetime is 1.47 ns, compared to 3.64 ns in the
pre-surgery data. We do not know where exactly this component comes from but it
must be from an anatomic structure in the front part of the eye. There is virtually
no such component in channel 2 (long wavelength channel).
Pre-Surgery Images, Channel 1
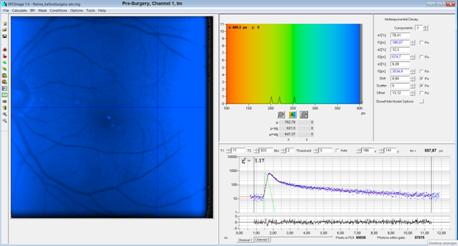
Fig. 5, pre-surgery,
tm image: Totally out of normal range, due to lens
fluorescence
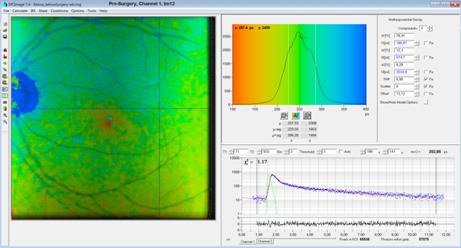
Fig. 6, pre-surgery
tm12 image: The lifetime is in the normal range, and close to the post-surgery
value. This is an indication that t1 and t2 are indeed
fundus-fluorescence components. Note the low contrast
due to the strong lens fluorescence.
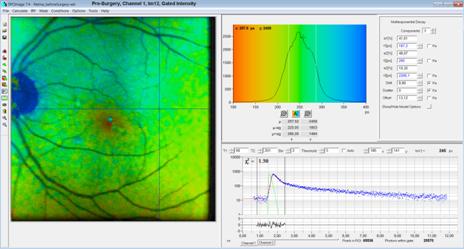
Fig. 7, pre-surgery
tm12 image, time-gated intensity: Increased contrast by
rejecting most of the lens fluorescence
Pre-Surgery Images, Channel 2
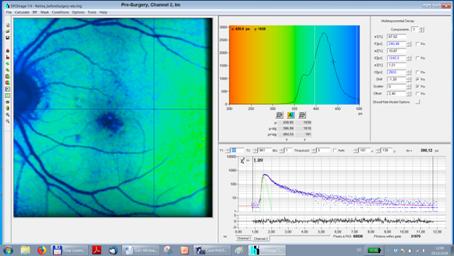
Fig. 8, pre-surgery
tm: Lifetime shifted due to lens fluorescence
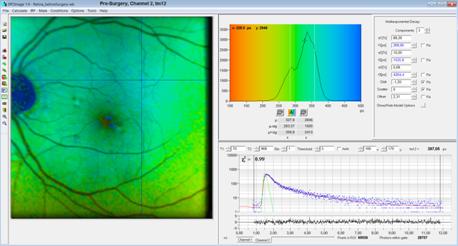
Fig. 9, pre-surgery
tm12: The lifetime is close to the post-surgery value (see
Fig. 13). This is an indication that t1 and t2 come from the fundus.
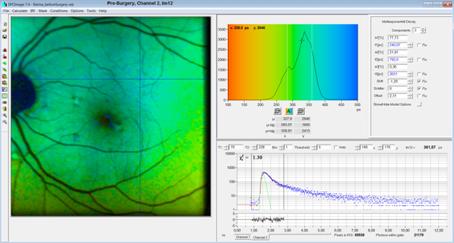
Fig. 10, pre-surgery
tm12, gated: Increased contrast by time-gated intensity
Post-Surgery, Channel 1
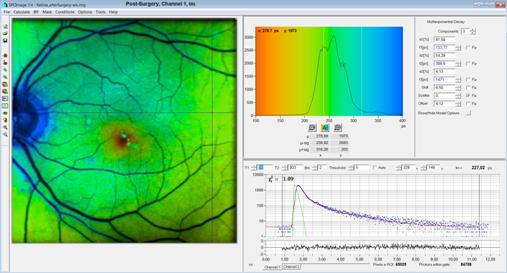
Fig. 11, post surgery tm image. A shifted slow component, t3, is still
detected. However, the lifetime (1471 ps) is different than that of the lens
(3600ps, see Fig. 6). It probably comes from other anatomic structures of the
front part of the eye.
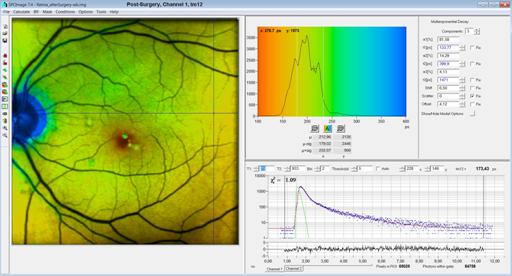
Fig. 12, post surgery tm12 image. In tm12,
the slow component, t3, is not included. Therefore tm12 is shorter that
tm. It is also shorter than tm12 of the pre-surgery image. It is possible that
the component is not present in the pre-surgery image, or that it is too week
to show up in the pre-surgery data.
Post-Surgery, Channel 2
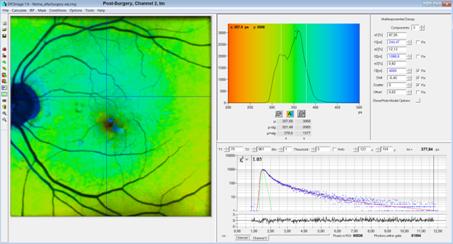
Fig. 13, post surgery tm image. The slow component, t3, is
extremely weak. A trace of a slow component, t3, turned up only by
fixing its lifetime, in this case to 4000ps.
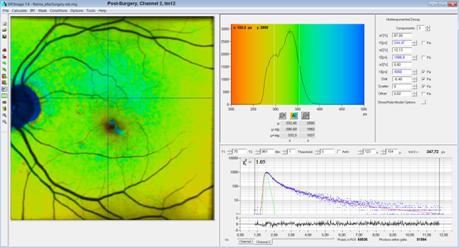
Fig. 14: post surgery tm12 image. Virtually no difference to Fig. 13
because the t3 component is extremely weak.
Summary
A new model function with a shifted third
component in combination with a fully syntethic IRF yields a substantial
improvement in the fit stability of FLIO data. We attribute the improvement to
the ability of the model to accurately fit the decay component from the front
part of the eye. This component is shifted in time and causes a step or a kink
in the rising edge of the fluorescence profiles. The accurate fit of the
leading part of the decay results in an accurate determination of the temporal
position of the IRF. This contributes significantly to the reproducibility of
the fit. Moreover, the ability to fit the early part of the decay profiles has
a direct influence on the accuracy at which fast fluorescence components are
determined. Importantly, the new model allows us the separate the fluorescence
decay components of the fundus from the decay component from the lens. This
works even for eyes with cataract where the lens is highly fluorescenct. As an
additional benefit, FLIO analysis with the new model runs virtually without
user interaction. Notably, it does not require tweaking the fit intervals or
fit parameters and thus avoids that the results are biased by the operator.
Acknkowledgements
The work described in this note was financially
supported by BMBF Germany, project 'Meta Netz'.
References
1. Karl M. Andersen, Lydia Sauer, Rebekah H. Gensure, Martin Hammer, Paul S. Bernstein,
Characterization of Retinitis Pigmentosa Using Fluorescence Lifetime Imaging
Ophthalmoscopy (FLIO). TVST 7 No. 3 (2018)
2. C. Dysli, G. Quellec, M. Abegg, M. N. Menke, U. Wolf-Schnurrbusch,
J. Kowal, J. Blatz, O. La Schiazza, A. B. Leichtle, S. Wolf, M. S. Zinkernagel,
Quantitative Analysis of Fluorescence Lifetime Measurements of the Macula Using
the Fluorescence Lifetime Imaging Ophthalmoscope in Healthy Subjects. IOVS 55,
2107-2113 (2014)
3. C. Dysli, M.Dysli, V. Enzmann, S. Wolf, M. S. Zinkernagel,
Fluorescence Lifetime Imaging of the Ocular Fundus in Mice. IOVS 55, 7206-7215
(2014)
4. C. Dysli, S. Wolf, K. Hatz, M. S. Zinkernagel, Fluorescence Lifetime
Imaging in Stargardt Disease: Potential Marker for Disease Progression. Invest
Ophthalmol Vis Sci. 57, 832-841 (2016)
5. Dysli, C., Wolf, S., Berezin, M.Y., Sauer, L., Hammer, M.,
Zinkernagel, M.S., Fluorescence lifetime imaging ophthalmoscopy, Progress in
Retinal and Eye Research (2017), doi: 10.1016/j.preteyeres.2017.06.005
6.
Dysli, C., Wolf, S., Berezin, M.Y., Sauer, L.,
Hammer, M., Zinkernagel, M.S., Fluorescence lifetime imaging ophthalmoscopy,
Progress in Retinal and Eye Research (2017), doi: 10.1016/j.preteyeres.2017.06.005
7.
C. Dysli, S. Wolf, M.S. Zinkernagel,
Fluorescence lifetime imaging in retinal artery occlusion. Invest Ophthalmol
Vis Sci. 2015; 56:33293336.
8.
C. Dysli, S. Wolf, H.V. Tran, M.S. Zinkernagel,
Autofluorescence lifetimes in patients with choroideremia identify
photoreceptors in areas with retinal pigment epithelium atrophy. Invest
Ophthalmol Vis Sci. 2016;57:67146721. DOI:10.1167/ iovs.16-20392
9.
C. Dysli, M. Dysli, M. S. Zinkernagel, V.
Enzmann, Effect of pharmacologically induced retinal degeneration on retinal
autofluorescence lifetimes in mice. Experimental Eye Research 153 (2016)
178e185
10.
C. Dysli, L. Berger, S. Wolf, M.N S.
Zinkernagel, Fundus autofluorescence lifetimes and entral serous
chorioretinopathy. Retina 37:21512161, 2017
11.
J.A. Feeks, J. J. Hunter, Adaptive optics
two-photon excited fluorescence lifetime imaging ophthalmoscopy of exogenous
fluorophores in mice. Biomed. Opt. Expr. 8(5), 2483-2495
12.
S. Jentsch, D. Schweitzer, K-U Schmidtke, S.
Peters, J. Dawczynski, K-J.n Bär, M. Hammer, Retinal fluorescence lifetime
imaging ophthalmoscopy measures depend on the severity of Alzheimers disease.
Acta Ophthalmologica (2014)
13.
M. Klemm, A. Dietzel, J. Haueisen, E. Nagel, M.
Hammer, D. Schweitzer, Repeatability of autoflurescence lifetime imaging at the
human fundus in healthy volunteers. Curr. Eye Res. 38, 793-801 (2013)
14.
Kwon S, Borrelli E, Fan W, Ebraheem A, Marion
KM, Sadda SR. Repeatability of Fluorescence Lifetime Imaging Ophthalmoscopy in
normal subjects with mydriasis. Trans Vis Sci Tech. 2019;8(3):15,
https://doi.org/10.1167/tvst.8.3.15
15.
Y. Miura, G. Hüttmann, R. Orzekowsky-Schroeder,
P. Steven, M. Szaszak, N. Koop, R. Brinkmann, Two-Photon Microscopy and
Fluorescence Lifetime Imaging of Retinal Pigment Epithelial Cells Under Oxidative
Stress. IOVS 54 j No. 6, 3369 (2013)
16.
Y. Miura, B. Lewke, A. Hutfilz, R. Brinkmann.
Change in Fluorescence Lifetime of Retinal Pigment Epithelium under Oxidative
Stress. Nippon Ganka Gakkai Zasshi (J Jpn Ophthalmol Soc) 123,105-114 (2019)
17.
L. Ramm, S. Jentsch, R. Augsten, M. Hammer,
Fluorescence lifetime imaging ophthalmoscopy in glaucoma. Graefes Arch Clin Exp
Ophthalmol (2014) 252:20252026
18.
Lydia Sauer, Rebekah H.
Gensure, PhD,1 Martin Hammer, Paul S. Bernstein, Fluorescence Lifetime Imaging
Ophthalmoscopy: A Novel Way to Assess Macular Telangiectasia Type 2.
Ophthalmology Retina 2 (6), 587-598 (2018)
19.
Lydia Sauer, Rebekah H. Gensure, Karl M.
Andersen, Lukas Kreilkamp, Gregory S. Hageman, Martin Hammer, Paul S. Bernstein,
Patterns of Fundus Autofluorescence Lifetimes In Eyes of Individuals With
Nonexudative Age-Related Macular Degeneration. IOVS 59 (2018)
20.
S. R. Sadda, E. Borrelli, W.g Fan, A. Ebraheem,
K. M. Marion, S. Kwon, Impact of mydriasis in fluorescence lifetime imaging
ophthalmoscopy. PLOS ONE | https://doi.org/10.1371/journal.pone.0209194
December 28, 2018
21.
L. Sauer, K.M. Andersen, B. Li, R.H. Gensure, M.
Hammer, P.S. Bernstein, Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO) of Macular
Pigment. Retina (2018)
22.
L. Sauer, Schweitzer D, Ramm L, Augsten R,
Hammer M, Peters S. Impact of macular pigment on fundus autofluorescence
lifetimes. Invest Ophthalmol Vis Sci. 2015;56:46684679.
23.
Lydia Sauer, Sven Peters, Johanna Schmidt,
Dietrich Schweitzer, Matthias Klemm, Lisa Ramm, Regine Augsten, Martin Hammer,
Monitoring macular pigment changes in macular holes using fluorescence lifetime
imagingophthalmoscopy. Acta Ophthalmologica 2016
24.
Johanna Schmidt, Sven Peters, Lydia Sauer,
Dietrich Schweitzer, Matthias Klemm, Regine Augsten, Nicolle Müller,Martin
Hammer, Fundus autofluorescence lifetimes are increased in non-proliferative
diabetic retinopathy. Acta Ophthalmologica 2016
25. D. Schweitzer, S. Schenke, M. Hammer, F. Schweitzer, S. Jentsch, E.
Birckner, W. Becker, Towards Metabolic Mapping of the Human Retina. Micr. Res.
Tech. 70, 403-409 (2007)
26. D. Schweitzer, M. Hammer, S. Jentsch, S. Schenke, Interpretation of
dymanic fluorescence of the eye. Proc. SPIE 677108-1 to -12 (2007)
27. D. Schweitzer,
S. Quick, S. Schenke, M. Klemm, S. Gehlert, M. Hammer, S. Jentsch, J. Fischer,
Vergleich von Parametern der zeitaufgelösten Autofluoreszenz bei Gesunden und
Patienten mit früher AMD. Der Ophthalmologe 8, 714-722
(2009)
28. D. Schweitzer, Quantifying fundus autofluorescence. In: N. Lois,
J.V. Forrester, eds., Fundus autofluorescence. Wolters Kluwer, Lippincott
Willams & Wilkins (2009)
29. D. Schweitzer, Metabolic Mapping. In: F.G. Holz, R.F. Spaide (eds),
Medical retina, Essential in Opthalmology, Springer (2010)
30. D. Schweitzer,
S. Quck, M. Klemm, M. Hammer, S. Jentsch, J. Dawczynski, Zeitaufgelöste
Autofluoreszenz bei retinalen Gefäßverschlüssen. Der
Ophthalmologe 12, 1145-1152 (2010)
31. D. Schweitzer, E.R. Gaillard, J. Dillon, R.F. Mullins, S. Russell,
B. Hoffmann, S. Peters, M. Hammer, C. Biskup, Time-Resolved Autofluorescence
Imaging of Human Donor Retina Tissue from Donors with Significant Extramacular
Drusen. IOVS, 53, 3376-3386 (2012)
32. D. Schweitzer, Autofluorescence diagnostics of ophthalmic diseases.
In: V.V. Ghukasyan, A.H. Heikal, eds., Natural biomarkers for cellular
metabolism. Biology, techniques, and applications. CRC Press, Taylor and Francis Group, Boca Raton, London, New York (2015)
33. D. Schweitzer, M. Hammer, Fluorescence Lifetime Imaging in
Ophthalmology. In: W. Becker (ed.) Advanced time-correlated single photon
counting applications. Springer, Berlin, Heidelberg, New York (2015
34. D. Schweitzer, Ophthalmic applications of FLIM. In: L. Marcu. P.M.W.
French, D.S. Elson, (eds.), Fluorecence lifetime spectroscopy and imaging.
Principles and applications in biomedical diagnostics. CRC Press, Taylor &
Francis Group, Boca Raton, London, New York (2015)
35. D. Schweitzer, L. Deutsch, M. Klemm, S. Jentsch, M. Hammer, S.
Peters, J. Haueisen, U. A. Müller, J. Dawczynski, Fluorescence lifetime imaging
ophthalmoscopy in type 2 diabetic patients who have no signs of diabetic
retinopathy. J. Biomed. Opt. 20(6), 061106-1 to 13 (2015)
36.
J. Teister, A. Liu, D. Wolters, N. Pfeiffer,
F.H. Grus, Peripapillary fluorescence lifetime reveals age-dependent changes
using fluorescence lifetime imaging ophthalmoscopy in rats. Exp. Eye Res. 176, 110-120 (2018)
37. W. Becker, The bh TCSPC handbook. Becker & Hickl GmbH, 7th ed.
(2017). Available on www.becker-hickl.com
38. Becker & Hickl GmbH, Sub-20ps IRF Width from Hybrid Detectors
and MCP-PMTs. Application note, available on www.becker-hickl.com
39. FLIO data acquisition and analysis. The road to success. Application
note in presentation-style, available on www.becker-hickl.com