We demonstrate two-photon FLIM with a FemtoFibre smart 780 laser of Toptica Photonics AG in combination with the Becker & Hickl DCS 120 MP TCSPC / FLIM system. The laser delivers femtosecond pulses at 783 nm, 80 MHz repetition rate, and 125 mW average power. We show that the laser power is sufficient to obtain high-resolution images in all the commonly performed FLIM applications. We demonstrate the performance of the system for label-free (autofluorescence) imaging of tissue, label-free imaging of cells, FLIM of ultrafast fluorescence decay in biological material, and FLIM of fluorophore-labelled cells and tissues.
Two-Photon FLIM with a Small Femtosecond Fibre Laser
Wolfgang Becker, Cornelia Junghans, Lukas Braun, Becker & Hickl GmbH
Alexander Jelzow, Toptica Photonics AG
We demonstrate two-photon FLIM with a FemtoFibre smart 780 laser of Toptica Photonics AG in combination with a Becker & Hickl DCS‑120 MP TCSPC / FLIM system. The laser delivers femtosecond pulses at 783 nm, 80 MHz repetition rate, and 125 mW average power. We show that the laser power is sufficient to obtain high-resolution images in all the commonly performed FLIM applications. We demonstrate the performance of the system for label-free (autofluorescence) imaging of tissue, label-free imaging of cells, FLIM of ultrafast fluorescence decay in biological material, and FLIM of fluorophore-labelled cells and tissues.
We have shown recently that two-photon FLIM of typical biological samples is possible with a 190 mW, 40 MHz femtosecond fibre laser [6]. The system delivered excellent results for label-free FLIM of tissue, cells, and small organisms. We also showed that conventionally labelled samples delivered excellent FLIM data, even when the laser wavelength didn't correspond exactly to the maximum of the absorption spectrum of the fluorophores. We were therefore tempted to try the same with the new 783 nm, 125-mW Toptica FemtoFibre smart 780 laser.
For the experiments we used an off-the-shelf FemtoFibre Smart [5] in combination with a bh DCS-120 MP FLIM system [1] and a Nikon TE 2000 inverted microscope. The photons were detected by two bh HPM-100-40 or -06 detectors [2, 7] attached to the microscope via a non-descanned (NDD) beam path [1, 6]. For general optical system configuration please see [6]. The data were recorded by two bh SPC-180NX TCSPC / FLIM modules [2] and analysed by bh SPCImage NG data analysis software [3]. The microscope lens was a Nikon x40 NA=1.3 oil immersion objective.
Label-Free Imaging
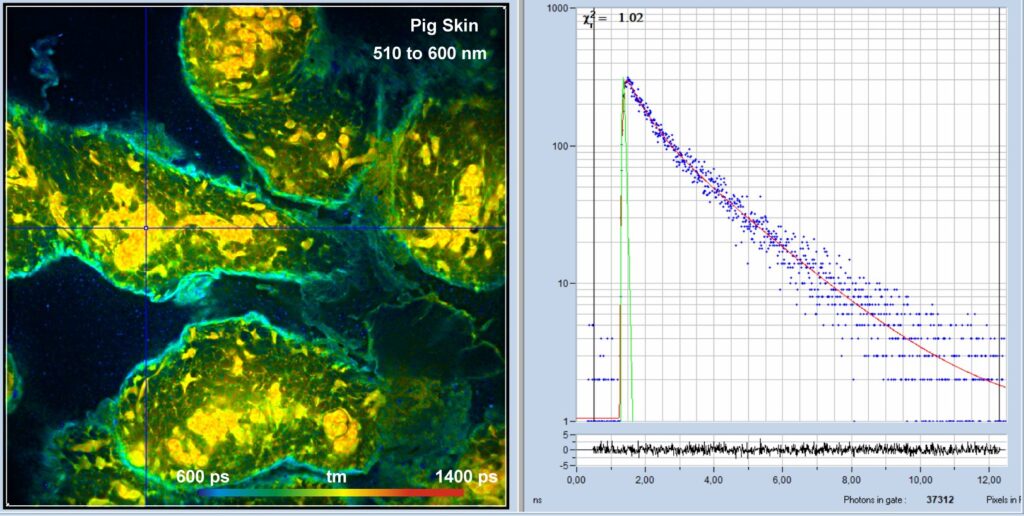
An NADH autofluorescence image of mammalian skin is shown in Fig. 1. The laser power in the sample plane was about 5 mW, the average count rate over the entire image 3×106 s-1.
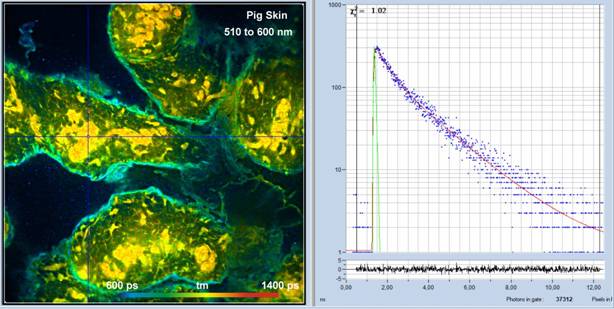
Fig. 1: Pig skin, autofluorescence, 510 to 600 nm. 1024 x 1024 pixels, 1024 time channels. Detection from 510 nm to 600 nm, amplitude-weighted lifetime, tm. Decay curve in 5x5 pixel area around the cursor position shown on the right.
Fig. 2 shows an image recorded in the second channel of the DCS-120 system simultaneously with the image in Fig. 1. The detection wavelength was 420 nm to 500 nm. The average count rate over the entire image was about 3×106 s-1.
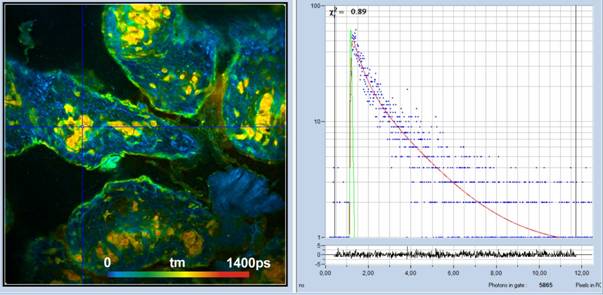
Fig. 2: Pig skin, autofluorescence, 420 to 500 nm Channel. 1024 x 1024 pixels, 1024 time channels. Detection from 520 nm to 600 nm, amplitude-weighted lifetime, tm, of double-exponential decay. Decay curve in 5x5 pixel area around the cursor position shown on the right.
FLIM of yeast cells is shown in Fig. 3. It was recorded in the detection channel from 420 nm to 500 nm. The laser power in the sample plane was about 5 mW. Higher power caused immediate damage in the sample. The count rate at 5 mW was about 400,000 s‑1.
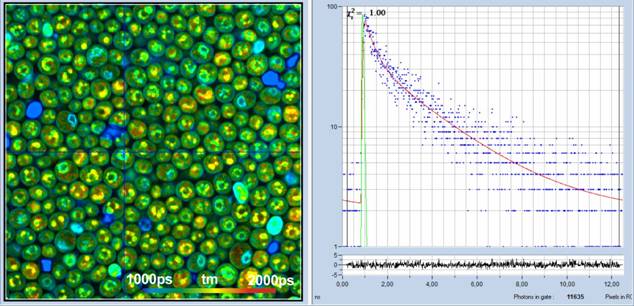
Fig. 3: Yeast cells, 420 to 500 nm, 1024 x 1024 pixels, 1024 time channels. Amplitude-weighted lifetime, tm, of double-exponential decay. Decay curve in 3x3 pixel area around the cursor position shown on the right.
Ultra-Fast Decay Processes
As a typical example of a sample with ultra-fast fluorescence decay, Fig. 4 shows a FLIM image of mushroom spores. To better resolve the fast decay the number of time bins was increased to 4096 channels, and the recording-time interval decreased to 2.5 ns. Time per channel under these conditions is 600 femtoseconds. The IRF width is about 18 ps fwhm, which was achieved by using the ultra-fast HPM-100-06 detector [2, 7]. The laser power was about 2 mW, the count rate about 350,000 s-1.Triple-exponential decay analysis at the cursor position delivers a mean lifetime, tm, of 33 ps, and a lifetime of the fastest component, t1, of 9 ps.
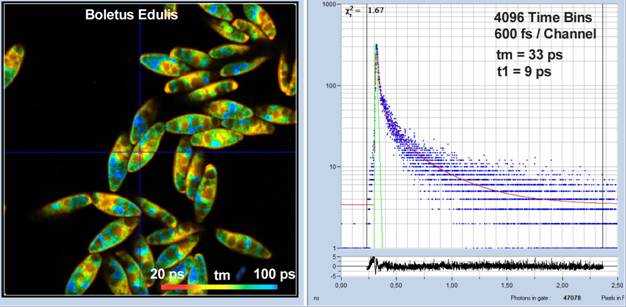
Fig. 4: Spores of Boletus edulis. 512 x 512 pixels, 4096 time channels, HPM-100-06 detector. Time bin width is 600 femtoseconds. IRF width is 18 ps fwhm. Decay curve shown on the right.
Samples Labelled With Standard Fluorophores
It could be argued that, with a fixed excitation wavelength of 783 nm, the system would be unable to excite commonly used fluorophores, such as DAPI, Alexa 488, Alexa 563, or Mito Tracker Red. This is, however not the case. Even if the excitation wavelength does not exactly match the absorption maximum of the fluorophore, there is always absorption on the short-wavelength side of the absorption maximum. Moreover, absorption spectra for two-photon excitation are usually broader than the corresponding one-photon spectra. The absorption at 783 nm is therefore sufficient to excite fluorescence bright enough to be detected by TCSPC FLIM. Examples for the excitation of commonly used fluorophores are shown in Fig. 5 and Fig. 6.
Fig. 5 shown BPEA cells labelled with Alexa 488, Mito Tracker Red, and DAPI. The images were recorded simultaneously in the two spectral channels of the DCS-120 FLIM system. With a laser power of 3 mW in the sample plane, bright fluorescence from all three fluorophores was obtained. The count rate in each channel was about 2×106 s-1 in the image on the left and 4×106 s-1 in the one on the right. Higher count rates can, in principle, be obtained by increasing the laser power. However, this causes visible photobleaching and changes in the average fluorescence lifetimes.
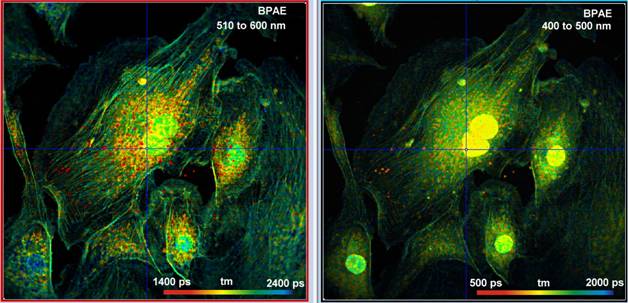
Fig. 5: BPAE cell labelled with Alexa 488, Mito Tracker Red, and DAPI. Power 3 mW in sample plane. Left: 510 to 600 nm, count rate 2×106 s-1. Right: 400 to 500 nm, count rate 4×106 s-1.
Fig. 6 shows a mouse kidney section labelled with Alexa 488, Alexa 568, and DAPI. Also here strong signals from all fluorophores were obtained. With a laser power of 3 mW in the sample plane the count rates in each channel were about 2×106 s-1.
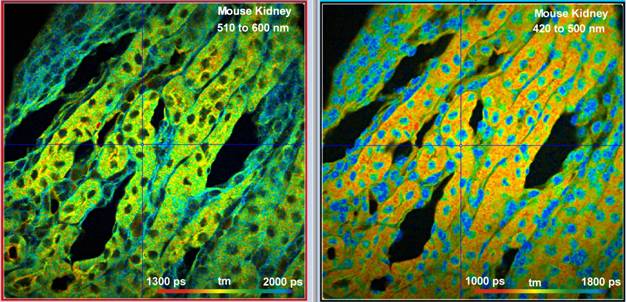
Fig. 6: Mouse kidney sample labelled with Alexa 488, Alexa 568, and DAPI. Images simultaneously detected in two spectral channels, 1024 x 1024 pixels, 1024 time channels. Excitation power 3 mW in the sample plane, count rate about 2×106 s-1 in each channel.
Technical Details
Microscope Lens
Both excitation efficiency and collection efficiency increases with the NA (numerical aperture) of the microscope lens. It is therefore recommended to use a lens with an NA as large as possible. In FLIM applications there is also another argument for large NA: An NA larger than one cancels the anisotropy decay component in the fluorescence decay functions [2]. It thus avoids the need of a magic-angle polariser and the associated loss in emission intensity. For the measurements presented in this paper we used a Nikon x40 NA=1.3 lens.
Excitation-Beam Diameter
The beam diameter in the plane of the microscope lens must be at least of the size of the lens back aperture. Smaller beam diameter leads to loss in spatial resolution, increase of focal volume, and, consequently, loss in excitation efficiency. The best resolution is not, as commonly believed, obtained by filling the lens aperture by a Gaussian beam but by illuminating it evenly over the entire diameter. This can only be achieved by over-illuminating it. Over-illumination has also the advantage that slight movement by the scanning process does not result in the beam moving off the aperture. This reduces the often encountered drop of intensity towards the edge of the field. To obtain even illumination of the back aperture the laser beam was expanded by a factor of two in front of the DCS-120 scan head. The back aperture of the Nikon x40 NA=1.3 lens was then over-illuminated by a factor of two. The resulting loss in excitation power is about a factor of four.
Excitation Power
With a 2x over-illumination of the microscope lens the maximum power in the sample plane was about 16 mW. It turned out that this is absolutely sufficient for all samples investigated. For most of the samples about 5 mW were the maximum that could be used without severe photobleaching or photodamage. An over-illumination factor of two was thus considered the best compromise between resolution and power reserve. Interestingly, the laser power required in the sample plane was no higher or even lower than in earlier experiments with the FemtoFibre PRO NIR laser [6]. This is surprising: The FemtoFibre smart 780 has 80 MHz repetition rate, as compared to 40 MHz of the FemtoFibre smart. This means it has only 1/2 of the peak power for a given average power, and therefore should yield half of the excitation efficiency. The fact that this is not the case means that either the pulse width of the FemtoFibre smart 780 is smaller or the beam quality is better.
Pixel Number
Except for the mushroom spores which were recorded with 512 x 15 pixels, all images were recorded with a pixel format of 1024 x 1024. These pixel numbers may appear extremely high for the standards of FLIM. In the past, most FLIM results were recorded with 256 x 256 pixels, and only recently bh have increased the default format to 512 x 512 pixels. However, 1024 x 1024 pixels appeared to be necessary to fully exploit the spatial resolution of the optical system. The decrease in the photon number per pixel is no longer important because it can be compensated for by overlapping binning in the lifetime analysis [9].
Acquisition Time
With the high pixel numbers used in our recordings the acquisition times for the images presented are not really short.
This is in part due to the large number of photon needed to fill all pixels with a reasonable number of photons, in part to the desire to obtain perfect images which are free of vissible photon noise. The acquisition time scales linearlily with the number of pixels. Thus, a 256 x 256 pixel image needs only 1/16 of the acquisition time of 1024 x 1024 pixel image to reach the same lifetime accuracy. The corresponding acquisition time would be 8 to 12 seconds, for medium-quality images even shorter.
Summary
The combination of the bh DCS-120 MP FLIM system with a Toptica FemtoFibre Smart laser makes a low-cost and small-size multiphoton FLIM system. The system is able to record high quality FLIM data in all commonly used FLIM applications. With its small size and its capability to record label-free (NADH) images it appears especially suitably for metabolic FLIM applications in hospitals [2, 8].
References
1. Becker & Hickl GmbH, DCS-120 Confocal and Multiphoton Scanning FLIM Systems, user handbook 9th ed. (2021). Available on www.becker-hickl.com
2. W. Becker, The bh TCSPC handbook. 10th edition (2023), available on www.becker-hickl.com
3. SPCImage NG data analysis software. In: W. Becker, The bh TCSPC handbook. 10th edition (2023)
4. Overview brochure, available on www.becker-hickl.com
Contact:
Wolfgang Becker
Becker & Hickl GmbH
Berlin, Germany
Nunsdorfer Ring 6-9
Email: becker@becker-hickl.com