Two-Photon FLIM
of Mushroom Spores Reveals Ultra-Fast Decay Component
Wolfgang Becker,
Cornelia Junghans, Axel Bergmann, Becker & Hickl GmbH
Abstract:
We performed FLIM on the spores of a variety of mushrooms that are commonly
found in the middle-European and north-American area. Using our DCS-120 MP FLIM
system with ultra-fast detectors, we found extremely fast components in the
decay functions. The decay times ranged from about t1
= 8 ps to 80 ps, with amplitudes a1 up to 99.5%. The decay
times and the amplitudes correlate with the colour of the spores. The darker
the spores are the more pronounced the fast component is. We attribute the
mechanism to an extremely efficient energy-transfer process, without being able
to tell what exactly the mechanism might be.
Motivation
Mushrooms belong to the oldest organisms on
earth. By recycling organic material, they are an integral part of the
ecological system. Moreover, many mushrooms live in symbiosis with trees. This
symbiosis is of vital importance not only for the mushrooms but also for the
trees, which do not grow well without the mushrooms. The spores of mushrooms
remain viable under harshest conditions. They can rise up to the stratosphere
and literally travel around the planet. Doing so, they survive heat, extreme
cold, extreme exsiccation, and UV radiation. The resistance of the spores to UV
radiation is particularly interesting. Considering the small size there is
essentially no conceivable way to efficiently block UV light from the inner
part of a spore. This raises the question whether there possibly is another
protection mechanism at work. A possible hint to that mechanism could be turned
up by fluorescence-lifetime measurements. We therefore performed FLIM on the
spores of a variety of mushrooms commonly found in Europe and North America. A
few examples are shown in Fig. 1.





Fig. 1: A selection of mushrooms the spores of which were investigated by
FLIM. Left to right: Amanita muscarina, Agaricus campestris, Coprinus comatus,
Boletus edulis, Hypholoma fasciculare.
Experiment Setup
From earlier experiments we knew that
fluorescence lifetimes of mushroom spores can be in the sub-100-ps range. Typical
one-photon confocal systems with diode-laser excitation then show an apparent
lifetime but do not reliably resolve the decay functions into individual decay components.
Therefore, we used our DCS-120 MP multiphoton system. The instrument is based
on fast beam scanning, two-photon excitation with a femtosecond fibre laser (Toptica
Femto Fibre Pro) and detection by ultra-fast hybrid detectors (bh HPM-100-06) [2,
4]. The data were recorded with by bhs multi-dimensional TCSPC technique (bh
SPC-150NX TCSPC FLIM modules). Please see [1, 2] for details. The instrument
response of the DCS-120 MP system is about 18 ps, full width at half
maximum. This is about 5 times faster than for a typical diode-laser based
system with GaAsP hybrid detectors, and 10 times faster than for FLIM systems
with conventional PMT detectors.
Sample Preparation and FLIM Procedure
Pieces of fresh mushroom caps were placed
over 180 µm thick microscope slides to collect the spores. For FLIM
measurement, the slides were placed on the sample stage of an inverted
microscope. To obtain maximum resolution and photon collection efficiency we
used a microscope lens with oil immersion, NA = 1.3 numerical
aperture, and 40x magnification. The emitted photons were recorded via the
non-descanned detection path of the DCS-120 MP system. A Chroma SP 700
short-pass filter was used to reject scattered laser light, and a 400-nm long
pass filter to suppress possible SHG light. Consequently, fluorescence was
detected from about 400 nm up to the upper detection limit of the
detector, which is about 650 nm.
We admit that, from the point of optics,
the configuration of the sample is not entirely correct. For best resolution,
the spores should be embedded in a medium with a refractive index close to 1.3.
However, attempts to image the spores in a solid environment failed because of
fluorescence of the embedding medium. Attempts to image the spores in water or
immersion oil failed because the laser beam induced motion in the sample. The
best and only way to run the experiments was to image the bare spores on the glass
from the back of the slide, as described above.
Another problem was that the spores,
especially those of brown or black colour, absorb at the fundamental wavelength
of the excitation laser. They are therefore easily destroyed by the laser. To
avoid any scanning artefacts, we kept the laser power below 2 mW, in some
cases even below 1.5 mW. Due to the low laser power the photon detection
rate was no higher than 200,000 counts per second for white spores and no
higher than 20,000 counts per second for dark spores. Consequently, the data
acquisition times were relatively long, typically in the range from one to ten
minutes. The long acquisition time was no problem, however, because our system
has excellent timing stability and a detector background count rate of no more
than 60 counts per second [3].
Results
Fig. 2 shows FLIM data of spores of Amanita
Muscarina. Amanita muscarina has white spores. A colour-coded image of the mean
lifetime (amplitude-weighted lifetime of triple-exponential model) is shown
left, a decay curve integrated over the pixels of a single spore is shown
right. The data do not show any surprise. The mean lifetime is in the range of 1 ns
to 2 ns. The lifetimes and amplitudes of the components are compatible
with NAD(P)H or FAD, or a mixture of both. No matter whether or not the fluorescence
comes from these compounds, the decay function is similar to the decay
functions found in other live matter.
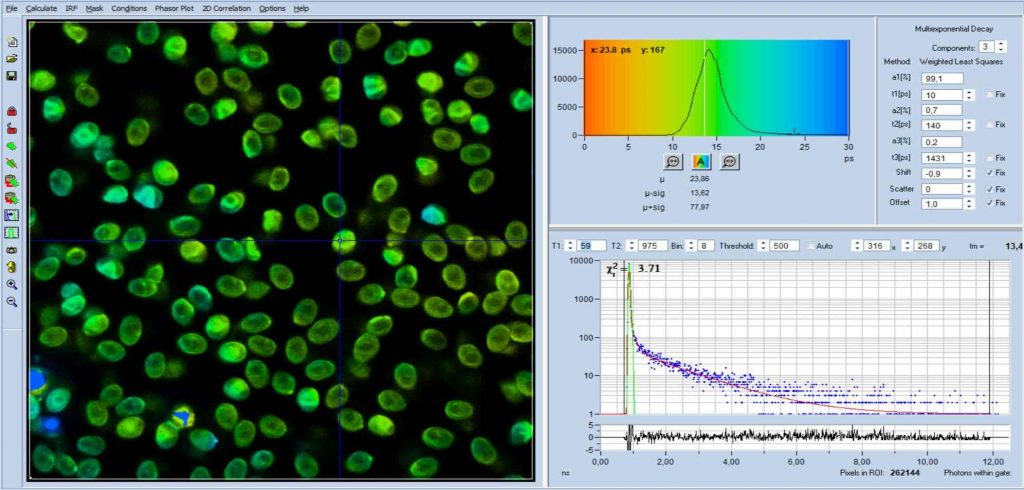
FLIM data of spores of Agaricus campestris
are shown in Fig. 3. In
contrast to Amanita muscarina, the spores of Agaricus campestris are dark
red-brown. The mean lifetime is in the sub-20 ps range. The real surprise
comes with the decay function. It is dominated by an ultra-fast component, with
a lifetime on the order of 10 ps, and an amplitude of 99.1%. The other two
components are almost entirely suppressed in favour of the fast component.
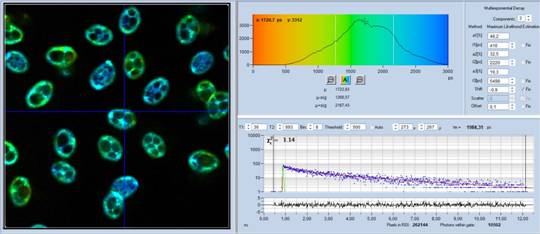
Fig. 2: Decay data of Amanita muscarina.
Lifetime image of mean decay time shown left, lifetime range 0 to 3000 ps.
Decay function shown lower right. lifetime histogram and decay parameters upper
right. Field size is approximately 80 x 80 µm.
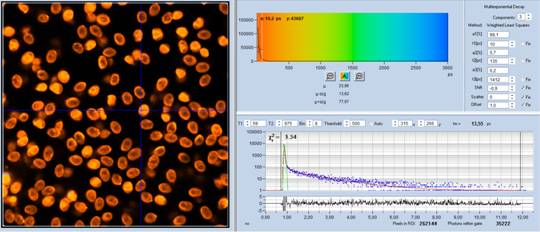
Fig. 3: Decay data of Agaricus campestris. Lifetime image of mean decay
time shown left, lifetime range 0 to 3000 ps. Decay function shown lower
right. lifetime histogram and decay parameters upper right. Field size is
approximately 125 x 125 µm.
It could be argued that the fast component
may be SHG or insufficiently blocked laser light. However, an additional laser
blocking filter in front of the detector neither changed the signal intensity
nor the shape of the decay curves. An additional long-pass filter with
450 nm cutoff wavelength reduced the photon rate but did not change the
decay curves noticeably. Therefore leakage of laser light or SHG can be excluded
as a source of the effect. The ultra-fast component is real.
To get a clue on the mechanism of the fast
emission we did FLIM on spores of a variety of other mushrooms. The results
revealed two interesting facts. First, there is a continuous transition from
the 'normal' decays to the decays with an ultra-fast component. Second, the lifetime
of the fast component is the lower and the amplitude the higher the darker the
spores are. Simultaneously, the intensity of the slow components decreases. The
results are listed in Table 1 and Table 2.
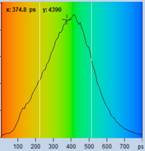
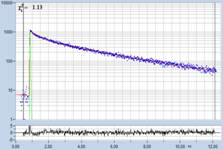

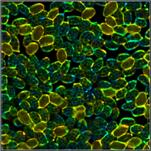
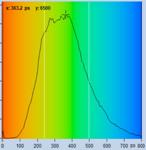
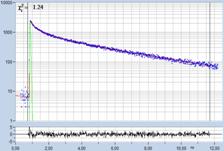

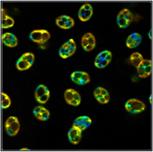
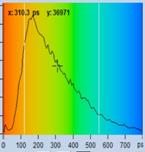
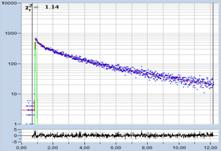

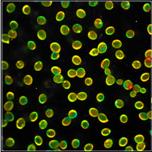
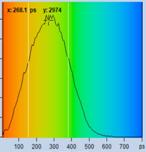
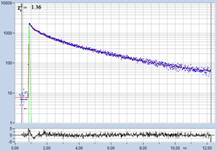

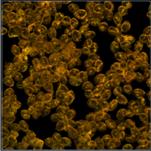
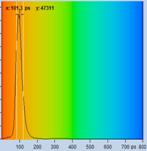
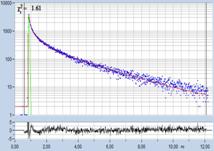

Table 1: Spores with lifetimes, t1, of the fast component from
360 ps to 100 ps, in order of decreasing t1. Left to right: t1
lifetime images (red to blue = 0 to 800 ps), distribution of t1 over
pixels, decay curve in selected spot, and text field describing species, spore
colour, t1 an a1, and zoom factor used for the recording. Field size is
500 µm / Zoom.
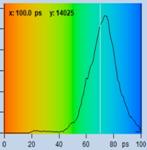
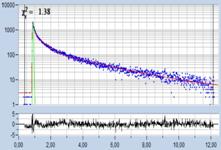
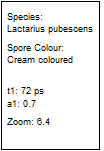
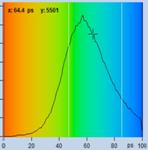
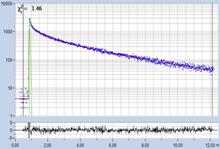

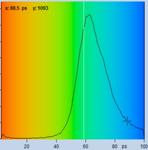
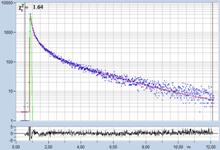

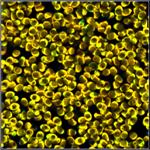
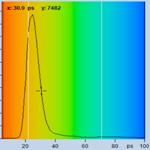
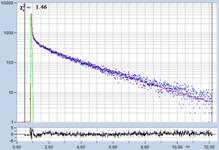

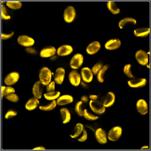
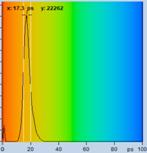
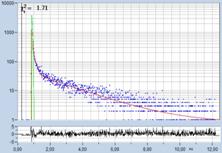

Table 2: Spores with lifetimes, t1, of the fast component below
100 ps, in the order of decreasing t1. Left to right: t1 lifetime images
(red to blue = 0 to 100 ps), distribution of t1 over pixels, decay curve
in selected spot, and text field describing species, spore colour, t1 an a1,
and zoom factor used for the recording. Field size is 500 µm / Zoom.
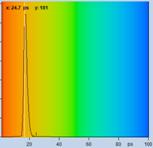
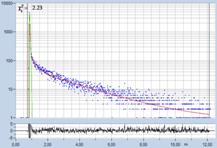

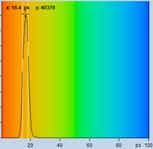
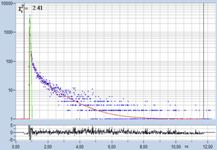
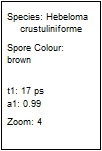
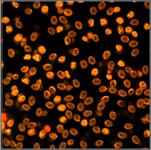
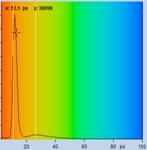


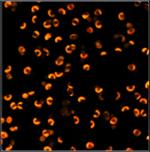
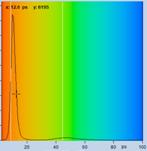



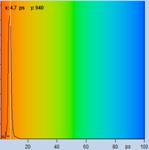
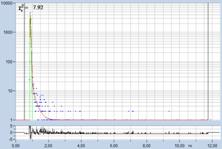

Table 2, continued: Spores with lifetimes, t1, of the fast component
below 100 ps, in the order of decreasing t1. Left to right: t1 lifetime
images (red to blue = 0 to 100 ps), distribution of t1 over pixels, decay
curve in selected spot, and text field describing species, spore colour, t1 an
a1, and zoom factor used for the recording. Field size is 500 µm / Zoom.
Interpretation of the Results
The decay functions found in these
experiments are unique and absolutely unusual. There is no known fluorophore
which has a fluorescence lifetime in the range of 10 to 20 ps. Such short
lifetimes can occur only if an extremely strong quenching process is at work.
This raises the question of what this process may be. In principle, one could
presume that the absorber present in the darker spores is fluorescent but
strongly quenched by some intramolecular deactivation process. The fact that
the fluorescence lifetime is related to the concentration of the absorber could
be explained by aggregation. The size of the aggregates certainly would depend
on the concentration of the absorber, and short lifetimes of aggregates are not
unusual. However, this model does not describe the observed decrease in
intensity of the slow lifetime components with increasing absorber
concentration. Of course, there should be an intensity drop simply due to
increasing absorption both for the excitation light and the fluorescence.
However, the intensity of the slow components decreases by several orders of
magnitude even for moderately coloured spores. Absorption is thus unlikely to
account for the full amount of intensity decrease.
An alternative model that describes the
results without these problems is inter-molecular energy transfer. Energy would
be transferred from the 'normal' fluorophores (acting as a donor) into a
non-fluorescent absorber (acting as an acceptor). The result would be a
quenching of the 'normal' fluorescence, causing a decrease in fluorescence
lifetime, and a decrease in intensity. The lifetime of the remaining
fluorescence would depend on the coupling efficiency. If not all of the
fluorophore molecules interact with the absorber (which is not unusual) some of
the original fluorescence would remain. The fraction of non-interacting
molecules would depend on the absorber concentration. This explains the
decrease in the amount of remaining 'normal' fluorescence with increasing absorber
concentration. Problems remaining are the extremely high energy transfer rate
needed to explain the short lifetime, and the gradual change in the decay time
of the fast component with the spore colour. If FRET is assumed to be the
source of the massive lifetime decrease the FRET efficiency must be on the
order of 0.99 and more. Such high FRET efficiencies have not yet been seen. The
gradual change in the lifetime of the fast component with the spore colour
requires that also the FRET efficiency changes gradually. This is no problem if
it is assumed that the absorbers in different species have different absorption
coefficients. Moreover, it is not unusual that the coupling efficiency gets
higher for higher acceptor concentration. The reason is that simultaneous interaction
with several acceptor molecules becomes more likely. Finally, there is possibly
another, even more elegant explanation. If the absorber increasingly forms
aggregates with increasing concentration the energy transfer efficiency could
increase dramatically, causing a similarly dramatic decrease in lifetime.
Altogether, this makes the energy transfer
mechanism more likely than the pure absorption mechanism. And, finally, if
nature has implemented a protection mechanism in mushroom spores it would
probably have developed one that not only blocks the light from the vital
constituents but also pulls out the energy from them.
A final decision between the two models
could be facilitated by spectral measurements. The problem of such measurements
is that there is no spectral FLIM detector which comes anywhere near to the
time resolution of the HPM-100-06 hybrid detector. Therefore, spectral
experiments have to be performed by subsequent measurements through different
narrow-band filters. These experiments will probably have to wait until the
next mushroom season.
References
1.
W. Becker, The bh TCSPC handbook. 8th edition
(2019), available on www.becker-hickl.com
2. Becker & Hickl GmbH, DCS-120 Confocal and Multiphoton Scanning FLIM
Systems, user handbook 8th ed. (2019). Available on www.becker-hickl.com
3. Becker & Hickl GmbH, The bh TCSPC Technique. Principles and
Applications. Available on www.becker-hickl.com.
4. Becker & Hickl GmbH, Two-Photon FLIM with a femtosecond fibre laser.
Application note, available on www.becker-hickl.com
Contact:
Wolfgang Becker
Becker & Hickl GmbH
Berlin, Germany
Email: becker@becker-hickl.com