- Complete Multiphoton FLIM Systems, Including Microscope and Laser
- Multiphoton FLIM Upgrades for Existing Conventional Microscopes
- Excitation by Ti:Sa or Femtosecond Fibre Laser
- Combined Multiphoton / Confocal Systems Available
- Laser Control Integrated in System Software
- Excitation Wavelengths from 650 nm to 1200 nm
- Scanning by Fast Galvanometer Mirrors
- Two Non-Descanned Detection Channels
- Channel Separation by Dichroic or Polarising Beamsplitters
- Recording by bh's Multidimensional TCSPC Process
- Two Fully Parallel TCSPC FLIM Channels
- Time Channel Width Down to 203 fs
- Ultra-Fast and Ultra-Sensitive Detectors
- Unprecedented Time Resolution
- Detection of Lifetimes <10 ps
- Near-Ideal Photon Efficiency
- Excellent Lifetime Reprodicibility
- Fast Online-FLIM
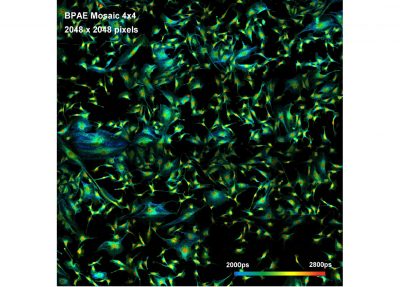
- Megapixel FLIM, 2048 x 2048 Pixels
- Precision FLIM, 4096 Time Channels
- Mosaic FLIM
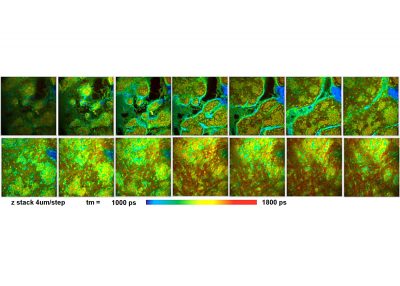
- Z Stack FLIM
- Accumulation of Fast Time Series
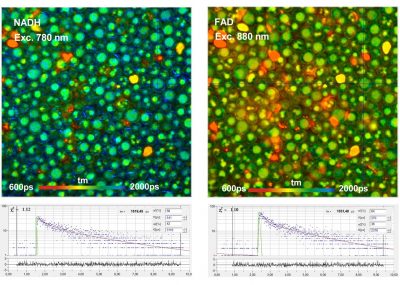
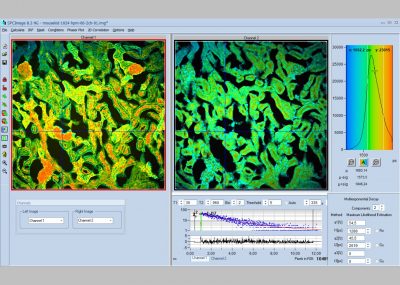
- Multi-Wavelength FLIM
- Simultaneous FLIM/PLIM
- Integrated Motorized Sample Stage
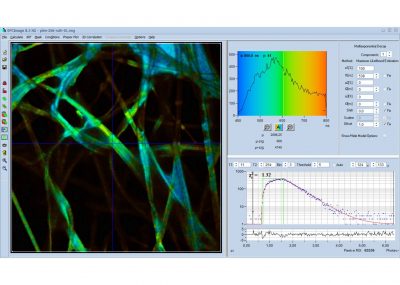
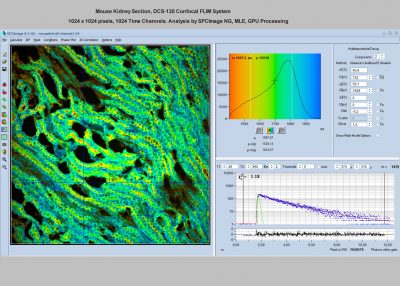
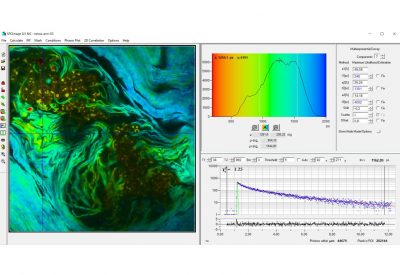
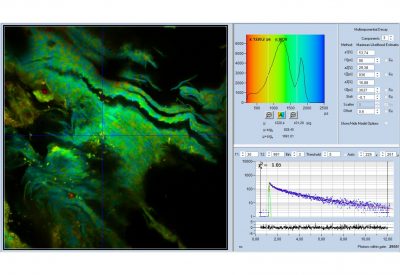
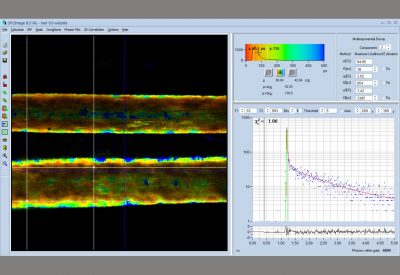
- Data Analysis by bh SPCImage NG
- Ultra-Fast Processing by GPU
- Combination of Time-Domain Analysis and Phasor Plot
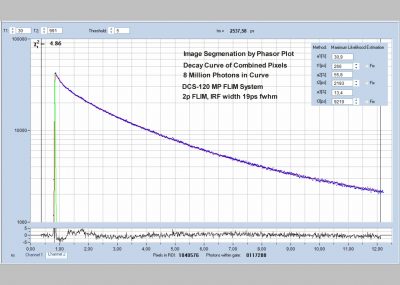
- Image Segmentation by Phasor Plot or 2D Histograms
- MLE Fit of Decay Curves
- Automatic IRF Modelling
- No Need to Record IRF
Description
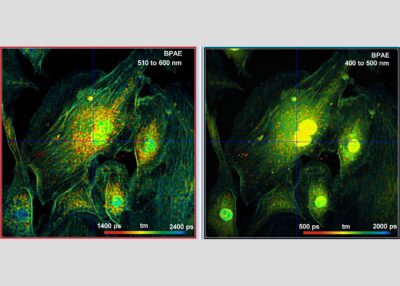
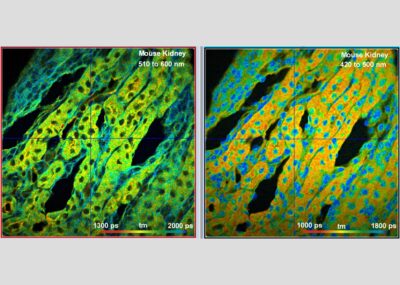
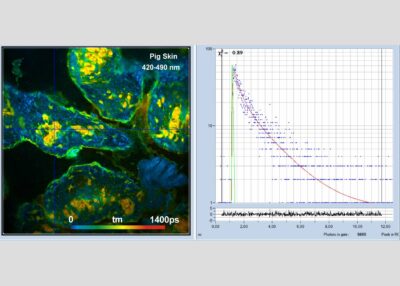
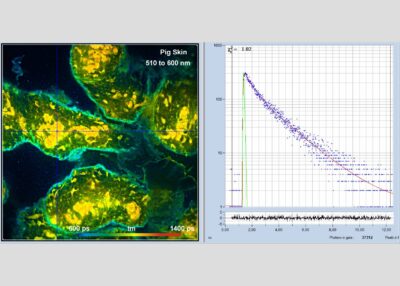
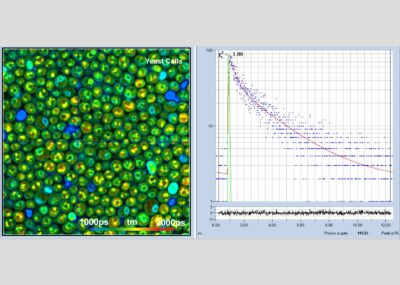
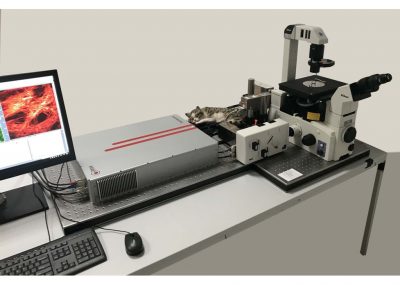
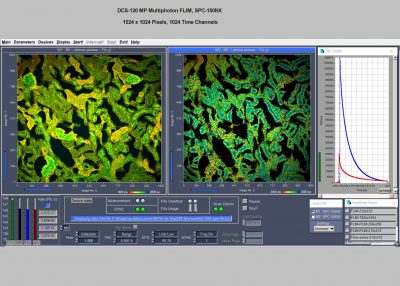
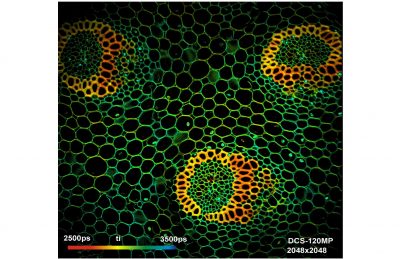
The DCS-120 MP system uses multiphoton excitation by femtosecond laser pulses, fast scanning by galvanometer mirrors, confocal detection, and FLIM by bh’s multidimensional TCSPC technique. It records fluorescence lifetime images at unprecedented temporal resolution, unprecedented reproducibility, high spatial resolution, high sensitivity, and near-ideal photon efficiency. Fluorescence lifetimes can be detected down to 10 ps; the decay data can be resolved into 4096 time channels of down to 203 fs width. The pixel format can be increased to 2048 x 2048.
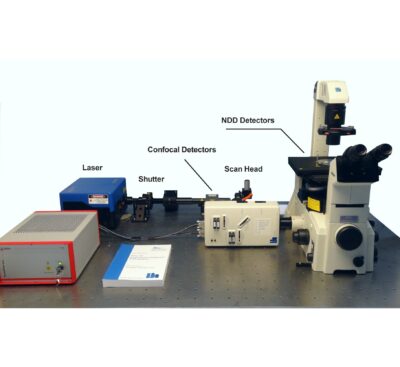
To make use of the high penetration depth of multiphoton excitation the DCS-120 MP used non-descanned detection. The MP system is available with inverted microscopes of Nikon, Zeiss, and Olympus. Due to its fast beam scanning and its high sensitivity the DCS-120 system is compatible with live-cell imaging. DCS-120 functions include simultaneous recording of FLIM or steady-state fluorescence images simultaneously in two fully parallel wavelength channels, laser wavelength multiplexing, time-series FLIM, time-series recording, Z stack FLIM, phosphorescence lifetime imaging (PLIM), fluorescence lifetime-transient scanning (FLITS) and FCS recording. Applications focus on lifetime variations by interactions of fluorophores with their molecular environment. Typical applications are ion concentration measurement, FRET experiments, metabolic imaging, imaging of fast physiological effects, and plant physiology.
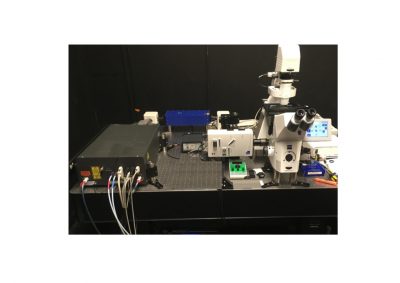
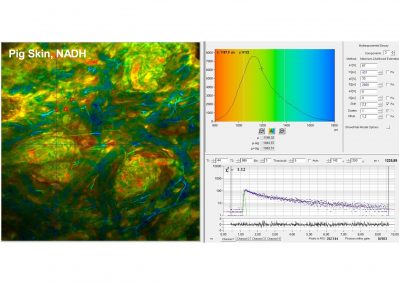
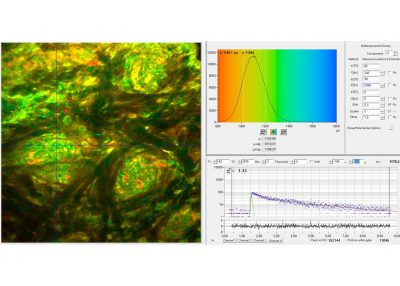
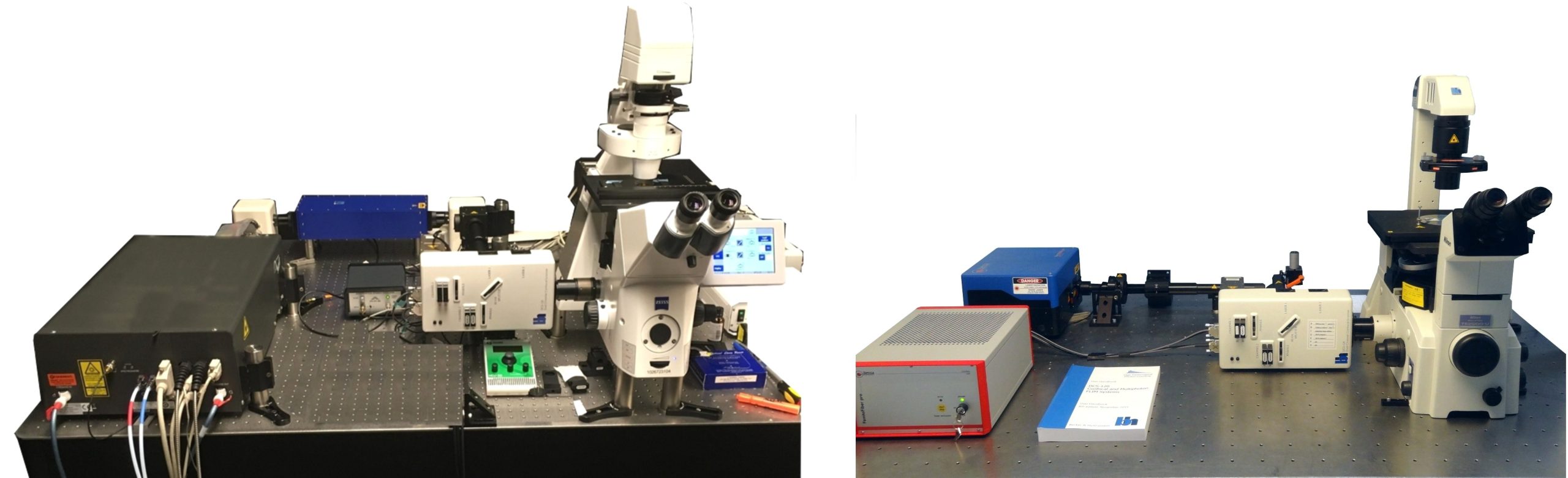
DCS-120 MP Multiphoton FLIM systems are available with titanium-sapphire lasers and with femtosecond fibre lasers. The two versions are shown in the figure below, left and right. Ti:Sa-laser systems have the advantage of tuneability and high laser power. Th high power allows the use of an AOM (acousto-optical modulator) for software-controlled intensity regulation, fast beam blanking, and pixel-synchonous on-off modulation for simultaneous FLIM /PLIM. Fibre-laser systems, on the other hand, are compact, easy to use, and virtually maintanance-free. On the negative side, the wavelength is fixed to typically 780 nm. However, the lack of tuneability is less of a problem than commonly believed: The two-photon-absorption bands of most fluorophores are broad enough to excite them at this wavelength. Moreover, 780 nm is an excellent wavelength to excite NADH. Fibre laser systems therefore make almost ideal multiphoton metabolic-FLIM systems.
Specifications
Selected Specifications
Principle: Scanning by fast galvanometers scanner, confocal detection, and TCSPC FLIM by bh's Multi-dimensional TCSPC technique
Excitation: Picosecond diode lasers
Scan Rate: Down to one microsecond per pixel
Buildup of Lifetime Images: Distribution of photons over arrival times and scan coordinates
Buildup of Fluorescence Correlation Data: Correlation of absolute photon times
General Operation Modes: FLIM in two spectral or polarisation channels, Multi-wavelength FLIM,
Time-series FLIM, Z-Stack FLIM, Mosaic FLIM, x,y, z, temporal, Excitation-wavelength multiplexed FLIM, FLITS (fluorescence lifetime-transient scanning), PLIM (phosphorescence lifetime imaging) simultaneously with FLIM, FCS, cross FCS, gated FCS, single-point fluorescence decay recording, single-point phosphorescence decay recording
Optical System: DCS-120 scan head
Optical Principle: Confocal, beam scanning by fast galvanometer mirrors
Laser Inputs: Two independent inputs, fibre coupled
Laser Power Regulation, Optical: Continuously variable via neutral-density filter wheels
Outputs to Detectors: Two outputs, detectors are directly attached
Main Beamsplitter Versions: Multi-band dichroic, wideband, multiphoton
Secondary Beamsplitter Wheel: Three dichroic beamsplitters, polarising beamsplitter, 100% to channel 1, 100% to channel 2
Pinholes: Independent pinhole wheel for each channel
Pinhole Alignment: Electronical, via piezo microstage
Pinhole Size: 11 pinholes, from about 0.5 to 10 AU
Emission Filters: Two filter sliders per channel in series
Connection to Microscope: Adapter to left side port or port on top of microscope
Coupling of Ti:Sa or fs Fibre Laser into Scan Head: Free beam, 1 to 2 mm diameter
Coupling of Additional ps Diode Laser: Single-mode fibre
Scan Controller: bh GVD-120
Principle: Digital waveform generation, scan waveforms generated by hardware
Frame Size: Frame scan 16 x 16 to 2048 x 2048 pixels, line scan 16 to 2048 pixels
X Scan: Continuous or pixel-by-pixel
Y Scan: Line by line
Laser Power Control, Electrical: Software, electrical signals to lasers
Laser Multiplexing: Frame by frame, line by line, or within one pixel
Beam Blanking: During flyback and when scan is stopped
Scan Rate: Automatic selection of fastest rate or manual selection
Scan Area Definition: Via zoom factor and offset, or interactive via cursors during preview
Fast Preview Function: 1 second per frame, 256 x 256 pixels
Beam Park Function: Via cursor in preview image or cursor in FLIM image
Laser Multiplexing: Frame, line, or pixel multiplexing
TCSPC System: bh Simple Tau or Power Tau TCSPC system
Number of Parallel TCSPC / FLIM Channels: 2
TCSPC / FLIM Modules: SPC-150NX or SPC-180NX
Electrical Time Resolution: 1.5 ps RMS / 3.5 ps FWHM
Minimum Time Channel Width: 405 fs
Timing stability Over 100 Seconds: Better than 0.8 ps RMS
Timing Stability Over 30 Minutes: Better than 5 ps RMS
Saturated Count Rate: 10 MHz per channel
Input from Detector: Constant-fraction discriminator
Reference (SYNC) Input from Laser: Constant-fraction discriminator
Synchronisation with Scanning: Via frame clock, line clock and pixel clock pulses
Scan Rate: Works with any scan rate
Synchronisation with Laser Multiplexing: Via routing function of TCSPC modules
Recording of Multi-Wavelength Data: Simultaneous in 16 channels, via routing function
Experiment Trigger Function: TTL, used for Z stack FLIM and microscope-controlled time series
Operation Modes of TCSPC System: Single f(t), oscilloscope, f(txy), f(t,T), f(t) continuous flow
FIFO (correlation / FCS / MCS) mode, Scan Sync In imaging, Scan Sync In with continuous flow
FIFO imaging, FIFO Imaging combined with with MCS imaging, mosaic imaging, time-series imaging
multi-detector operation, laser multiplexing operation, cycle and repeat function, autosave function
Display Functions (Online): Intensity images, gated intensity images, lifetime images, decay curves in ROIs, decay curves, FCS curves, intensity traces.
No. of Images Displayed Simultaneously: 8
Max. Image Formats, Pixels X x Pixels Y x Time Channels: 2048 x 2048 x 256, 1024 x 1024 x 1024, 512 x 512 x 4096, 256 x 256 x 4096
Software:
Data Acquisition Software: bh SPCM
Scanner Control Software: Integrated in SPCM
Operating System: Windows 10 64 bit
Data Analysis Software: bh SPCImage NG
Principle of Data Analysis: MLE with GPU processing
Model Functions: Single, double, triple exponential decay, single, double, triple exponential decay with incomplete decay, shifted component model.
IRF Modelling: Syntethic IRF function fit to decay data
Excitation Sources
Multiphoton FLIM: Ti:Sa laser or fs fibre laser
Confocal FLIM: One additional BDS-SM ps diode laser can be coupled in the system.
Detectors:
NDD Detectors: Coupled directly to microscope
Confocal Detectors: Coupled directly to scan head
Standard Detectors: HPM-100-40 hybrid detector with GaAsP cathode, 300 to 720 nm
Optional: HPM-100-06 detector with <20 ps FWHM IRF width, 300 to 650 nm
Optional: HPM-100-50 detector, 400 to 900 nm
Optional: MW-FLIM GaAsP multiwavelength detector
For Complete Specifications Please See:
DCS-120 Confocal and Multiphoton FLIM Systems, User Handbook
Downloads
Documents
Documents
- DCS-120 Confocal and Multiphoton FLIM Systems – Overview Brochure
- FLIM Systems for Laser Scanning Microscopes – Overview Brochure
- Bigger and Better Photons: The Road to Great FLIM Results
- SPCImage NG – Overview Brochure
- The bh FLIM Technique – More than Lifetime Imaging
- DCS-120 Confocal and Multiphoton FLIM Systems – User Handbook 9th ed. 2021
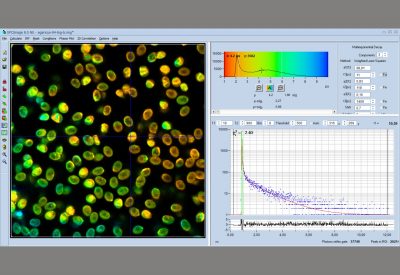
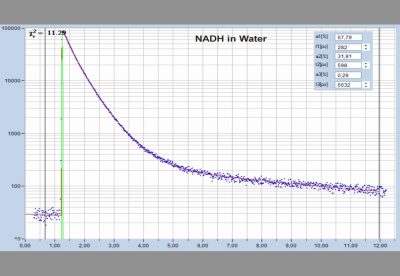
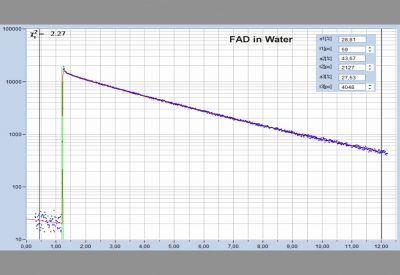
The realm of the bh FLIM systems is in molecular imaging. Typical applications are the imaging of ion concentrations, pH, or local viscosity, protein interaction experiments by FRET, and metabolic imaging by fluorescence decay of NADH an FAD in combination with oxygen measurement. In these applications, the bh FLIM systems benefit from their high sensitivity, high time resolution, high timing stability, and their capability to resolve multi-exponential-decay profiles into their components. Other advantages are the capability to record FLIM of fast physiological effects down to the millisecond range, and to record at several excitation and emission wavelengths simultaneously.
Applications
Application Notes
- Recording the Instrument Response Function of a Multiphoton FLIM System
- Non-Descanned FLIM Detection in Multiphoton Microscopes
- Microsecond Decay FLIM: Combined Fluorescence and Phosphorescence Lifetime Imaging
- DCS-120 Confocal Scanning FLIM System: Two-Photon Excitation with Non-Descanned Detection
- Mosaic FLIM: New Dimensions in Fluorescence Lifetime Imaging
- Simultaneous Phosphorescence and Fluorescence Lifetime Imaging by Multi-Dimensional TCSPC and Multi-Pulse Excitation
- DCS-120 FLIM System Records X-Y Mosaics
- DCS-120 MP System Records Multiphoton FLIM and PLIM
- Two-Photon FLIM with a Femtosecond Fibre Laser
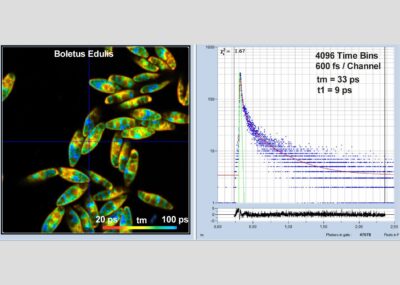
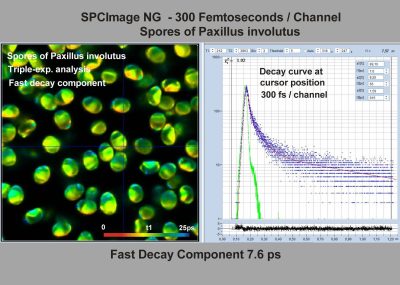
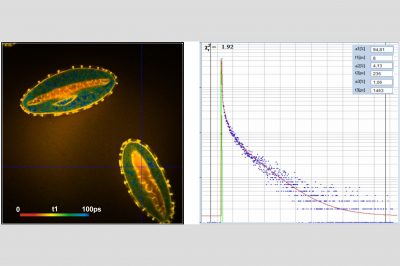
- Two-Photon FLIM of Mushroom Spores Reveals Ultra-Fast Decay Component
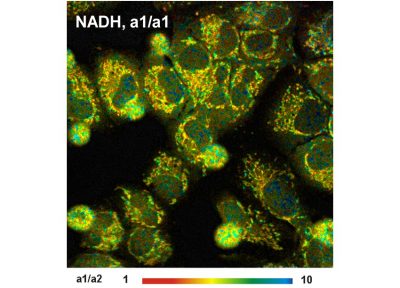
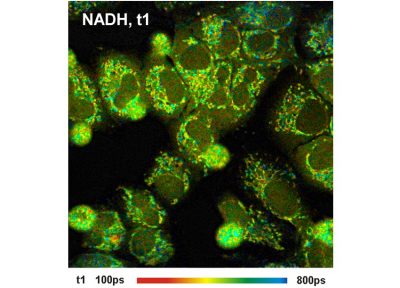
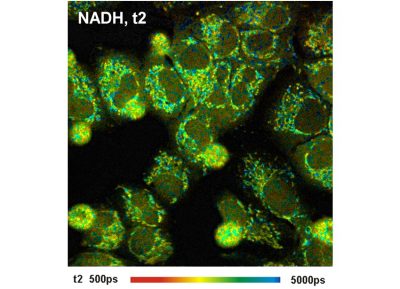
- High-Resolution Measurement of NADH and FAD Fluorescence Decay with the DCS-120 MP
- Two-Photon FLIM of Pollen Grains Reveals Ultra-Fast Decay Component
- FLIM at a Time-Channel Width of 300 Femtoseconds
- Ultra-Fast Fluorescence Decay in Scottish Whisky
- Ultra-Fast Fluorescence Decay in Natural Carotenoids
- Ultra-Fast Fluorescence Decay in Malignant Melanoma
- Label-Free Multiphoton FLIM of Moving Bacteria
- Megapixel FLIM with bh TCSPC Modules – The New SPCM 64-bit Software
- New SPCImage Version Combines Time-Domain Analysis with Phasor Plot
- High-Resolution Multiphoton FLIM Reveals Ultra-Fast Fluorescence Decay in Human Hair
- Two-Photon FLIM System with a Small Femtosecond Fibre Laser
- Metabolic FLIM with Simultaneous pH Imaging
Principles
Multiphoton Excitation
Multiphoton excitation uses two or more photons of the excitation light to excite one fluorescence photon, see figure below, left. To get the process working at noticeable efficiency extremely high power density, both in space and in time, is required. Both are achieved by using femtosecond pulses and focussing through a high-NA microscope objective lens, see below, right. With a pulse duration of 100 fs, a repetition rate of 80 MHz, and focusing through a lens of NA = 1.3, no more than 5 mW are required to obtain bright fluorescence of most fluorescent dyes used in microscopy. In most instances 'multiphoton' excitation is performed by using just two photons. To obtain fluorescence in the visible region the laser wavelength must then be in the near-infrared (NIR) region. Ti:Sa lasers, OPOs, and femtosecond fibre lasers can be used to generate the required excitation wavelength.
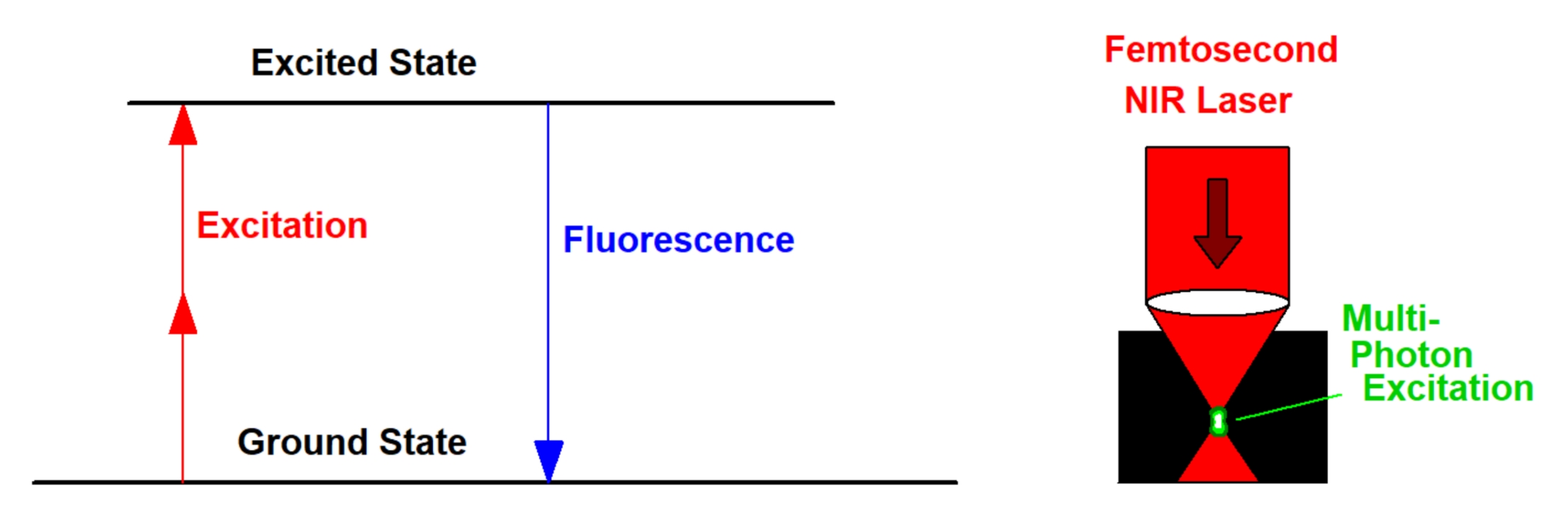
Multiphoton excitation has a number of advantages over one-photon excitation.
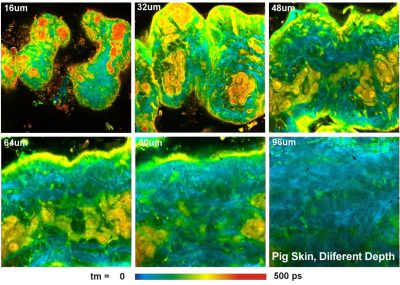
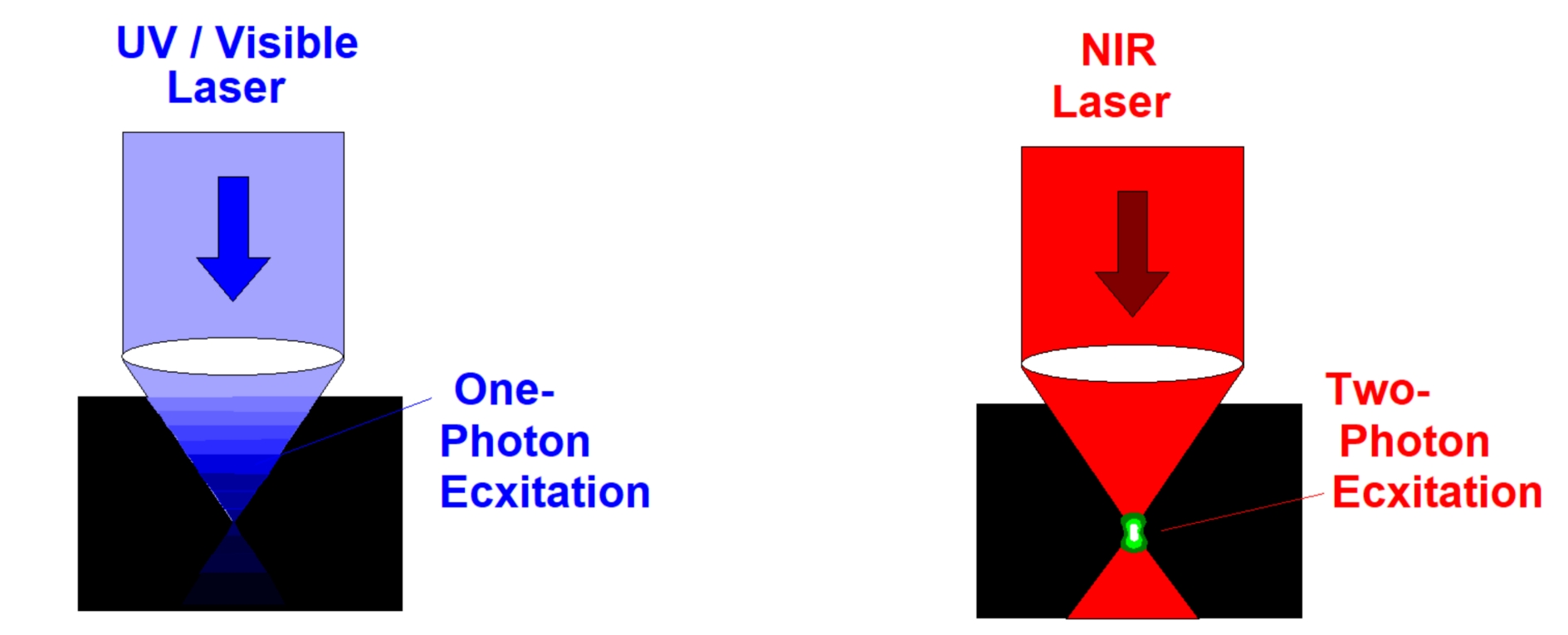
First, the absorption of the sample at the excitation wavelength is low. Also the scattering coefficient is lower than at visible or ultraviolet wavelengths. Two-photon (or, in the general case, multiphoton) excitation therefore goes deeper into tissue than one-photon excitation, see figure below, left and right. Multiphoton excitation can therefore be used to excite fluorescence in deep layers of biological tissue.
Second, the excitation is confined to the focal volume, where the power density is high. In a scanning system, this automatically implies that the excitation is confined to the focal plane. That means no confocal pinhole is needed to reject light from outside the focal plane. Multiphoton excitation intrinsically provides out-of-focus suppression and, consequently, optical-sectioning capability.
The fact that no fluorescence is excited outside the focal plane leads to the third advantage of multiphoton excitation, and this is detection of light scattered inside the sample. Visible light from a deep focal plane is massively scattered on its way out of the sample, see figure below, left. This light cannot be collimated, and, consequently, not focused into a confocal pinhole. However, there is no need to feed the light back through the scanner and through a pinhole. Instead, it can be diverted immediately behind the microscope lens and sent to a detector. By projecting an image of the microscope lens (not of the image plane in the sample) a large part of the scattered photons is transferred to the detector. The principle is called non-descanned detection, see figure below, right.
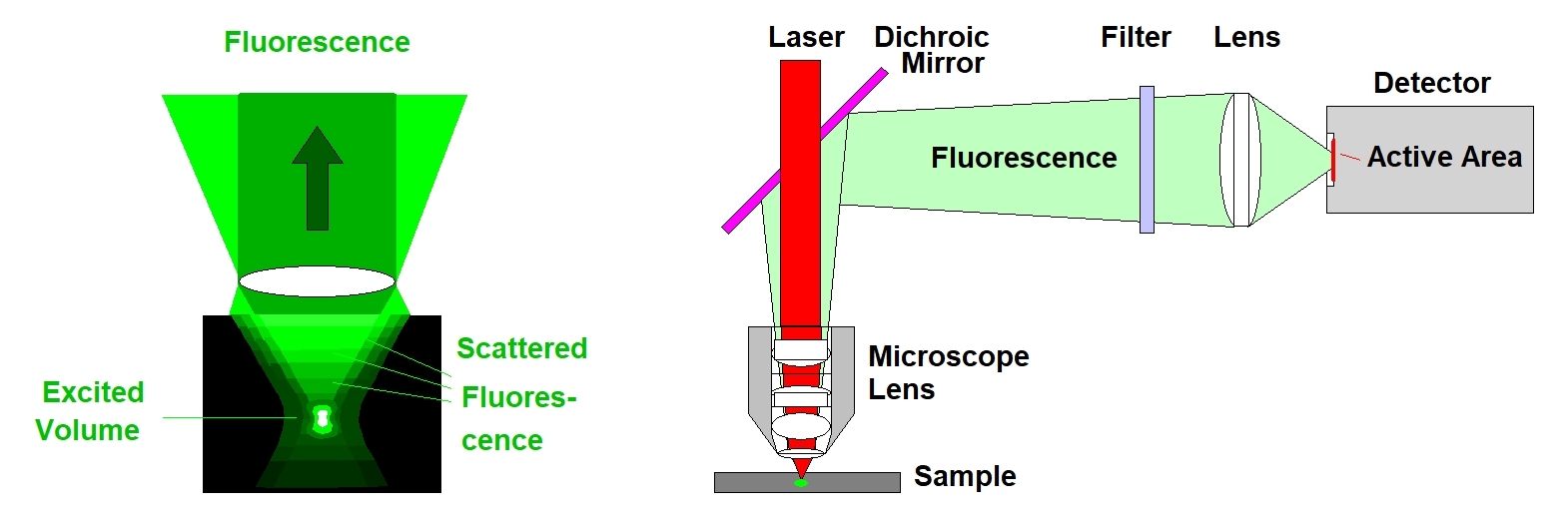
In combination with the bh TCSPC FLIM systems a fourth advantage is the extremely high time resolution. The excitation pulses have femtosecond pulse width. The instrument-response function (IRF) of the FLIM system is therefore not broadened by the excitation pulses. With fast hybrid detectors, IRF widths down to 20 ps (fwhm) are reached. The capability to directly record ultra-fast fluorescence decay phenomena opens a entirely new world of FLIM applications. Please see application notes about ultra-fast decay in mushroom spores and in pollen grains, and its measurement in the sub-10-ps range.