FLIM at a Time-Channel Width of 300 Femtoseconds
Wolfgang Becker, V.
Shcheslavskiy, Axel Bergmann
Becker & Hickl
GmbH
Abstract:
For many years, TCSPC FLIM
has been performed with 256 time channels and a time-channel width on the order
of 50 ps. With the introduction of faster detectors the number of time channels
was increased to 1024 and the channel width decreased to 10 ps. Recently, bh
have introduced detectors with sub-20 ps IRF width. The Nyquist criterion
suggests that FLIM data with these detectors should be recorded with a channel
width of about 2 ps. To extract ultra-fast decay components hidden in the
detector response even smaller time-channel width can be useful. Here, we
report on extracting ultra-fast decay components from FLIM data recorded with a
time-channel width of 300 femtoseconds.
Time-Channel width of TCSPC FLIM
For many years TCSPC FLIM data were
recorded at a resolution of 256 time channels on the time axis [1]. At a
typical observation-time interval of 12 ns 256 time channels gave a
time-channel width of about 50 ps. Detectors used at this time, such as
the Hamamatsu H7422-40 or the Hamamatsu H5773 and H5783 delivered IRF widths of
about 300 ps and 180 ps full width at half maximum (fwhm),
respectively [1]. With 50-ps channels, the instrument response functions (IRFs)
of these detectors were considered to be sufficiently sampled to fulfil the
Nyquist criterion. Under Nyquist conditions, the result of FLIM data analysis
does not depend on the relative location of the data points on the IRF or on
the rising edge of the fluorescence pulse. Higher numbers of time channels were
therefore considered 'empty' resolution, wasting only data space and
data-analysis time.
The requirements changed with the
introduction of the bh HPM-100-40 hybrid detectors [1, 2, 3]. FLIM systems with these detectors deliver
a system IRF of 100 to 140 ps, fwhm, depending on the excitation pulse
width. bh therefore moved to a standard FLIM format of 1024 time channels. The
time-channel width for a 10-ns recording interval is then about 10 ps.
That means the IRF is sampled with 10 or more data points, and the Nyquist
criterion is, again, satisfied. Of course, the four-fold increase in the number
of time channels leads to a similar increase in data size, and, consequently,
in FLIM data processing time. bh therefore implemented GPU processing in the
data analysis software. GPU processing speeds up the data analysis by a factor
of 100 so that data processing time is no longer a problem [4].
Recently, bh have introduced FLIM systems
with SPC-150NX and HPM-100-06 detectors [1, 5]. With femtosecond excitation, these systems
deliver IRF widths smaller than 20 ps, fwhm [6]. That means a time-channel width of
2 ps or less should be used to satisfy the Nyquist criterion, and the number
of time channels should be increased to 4096. It could be objected that
distributing the photons over a number of channels this large would result in a
smaller number of photons per channel and, consequently, in a decrease of the accuracy
of the FLIM data analysis. This is, however, not the case. The signal-to-noise
ratio of the lifetime is independent of the number of time channels, as has
been shown in [7]. It only depends on the total number of photons. Three
examples of FLIM decay traces recorded with different FLIM data formats are
shown in Fig. 1.
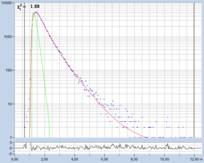
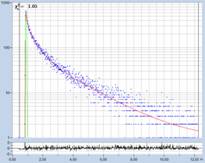
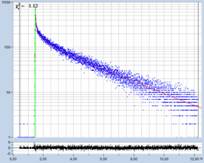
Fig. 1: Decay curves from FLIM data with different time-channel width.
Left to right: 50 ps, 12 ps, and 2.4 ps. Recording-time interval
12.5 ns. The red curve is a fit with a three-component model. (Data from
different samples)
A time-channel width on the order of
2 ps does not pose a problem to the bh TCSPC / FLIM modules - it
can even be reached with early modules, such as the SPC-830. However, just covering
the IRF width with enough data points is not all. High-resolution FLIM
systems may be used to extract ultra-fast decay components with lifetimes
shorter than the IRF width. Such decay components are more frequent than
commonly believed. We have found them in mushroom spores, plant tissue,
mammalian hair, and in malignant melanoma [8, 9, 10]. The components can have decay
times down to 7 ps, perhaps even less. In these cases not only the IRF but also
the decay function must be adequately sampled. Consequently, time-channel
widths below 700 fs should be used in these cases. Channel widths in this
range are available from the SPC-180NX, the SPC-180NXX, and the equivalent
SPC-150NX and -NXX versions [1].
Effect of the Channel Width on the Resolution of the Decay
Components
To demonstrate the effect of different
time-channel width on the resolution of fast decay components we recorded lifetime
images of mushroom spores with our DCS-120 MP fibre-laser multiphoton system [6].
The detector was a HPM-100-06 module, the TCSPC module an SPC-180NXX. Two-photon
images at 780 nm excitation wavelength were taken from spores of Paxillus
involutus, IRFs were recorded by taking SHG images of finely powdered
sugar. All data were recorded through a non-descanned detection path. Scattered
laser light was blocked by Chroma SP680 filters. The emission filter for
the FLIM recordings was a 450-nm long pass. For IRF recording the filter was
taken out. Pairs of fluorescence images and SHG images were recorded with a
time-channel width of 3 ps and 300 fs. The total number of time
channels was 4096 in both cases, covering observation-time intervals of
12.5 ns and 1.25 ns, respectively. Raw data from SPCM data
acquisition software are shown in Fig. 2. An ultra-fast decay component is
clearly visible in the data.
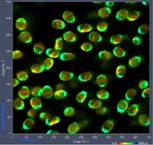

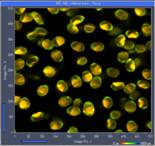
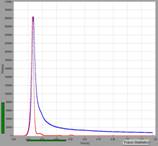
Fig. 2: Paxillus involutus spores, raw data in SPCM. Images, decay
curves (blue) and IRFs (red), 3 ps / channel and
300 fs / channel. Displayed by online display functions of SPCM
data acquisition software, linear scale.
Precision FLIM analysis was performed by bh
SPCImage NG FLIM data analysis software [1, 4]. In all cases,
triple-exponential decay analysis was applied to the data. Data recorded with
3 ps channel width are shown in Fig. 3. An image of the fastest decay
component, t1, is shown on the left, a decay curve at the cursor position in
the middle. The insert in the decay window shows the amplitudes and the
lifetimes of the decay components. A histogram of the t1 values over all pixels
of the image is shown on the right.
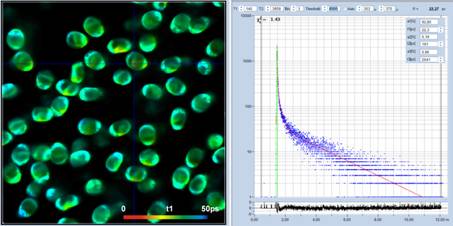
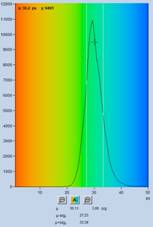
Fig. 3: Spores of Paxillus involutus, time-channel width 3 ps,
4096 time channels, observation-time interval 12.5 ns. Left to right:
Image of fastest decay component, t1, decay curve at cursor position (blue) and
IRF (green), histogram of t1 values over the pixels of the image. Auto IRF of
SPCImage NG.
As can be seen from the figure, a good fit
of the decay data is obtained. The lifetime of the fast component, t1, is obtained
at high signal-to-noise ratio, as the t1 image and the histogram show. The most
frequent value of t1 is about 30 ps.
For the analysis shown above the 'auto' IRF
of SPCimage was used, please see [1], chapter 'SPCImage NG Data Analysis
Software'. Experience has shown that for extremely fast decays the 'auto' IRF
often comes out a bit too short. Therefore we analysed the same data with a
real (measured) IRF. The result is shown in Fig. 4. The figure shows that the
general composition of the decays remains the same, with the difference that t1
becomes a bit shorter. However, considering the fact that the lifetime is on
the on the order of only 25 ps, the difference is not significant.
Nevertheless, analysis of further data with femtosecond time-channel width was
performed with the real IRF.
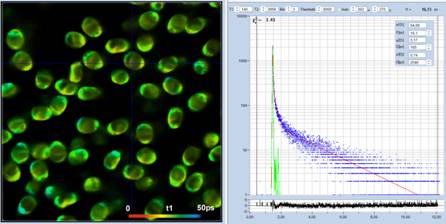
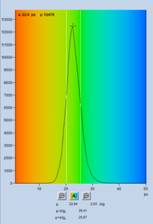
Fig. 4: Same
as in Fig. 3, but analysed with the real IRF.
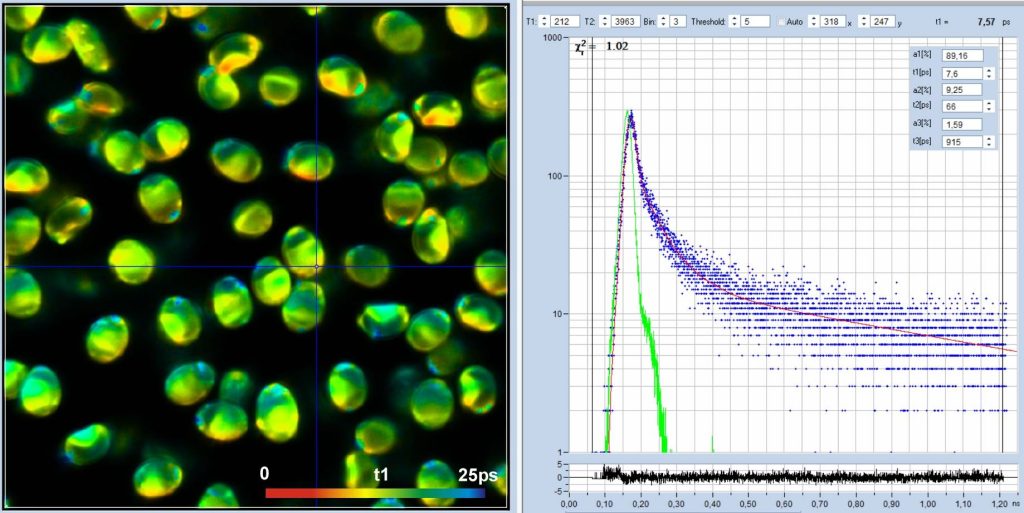
Fig. 5 shows data recorded with a
time-channel width of 300 femtoseconds, i.e. on a time scale 10 times faster
than in Fig. 3 and Fig. 4. The number of time channels is 4096, the total observation-time
interval 1,25 ns. The decay data in a selected spot are shown in the
middle. The green curve is the IRF, the blue dots are the photon numbers in the
time channels. The IRF is a real one, recorded from powdered sugar. At the time
scale used, the fwhm of the IRF extends over 70 data points. The figure
resembles a decay/IRF plot from a conventional lifetime spectrometer, with the
difference that the time scale is 20 times faster. With the data resolved into
a number of data points this high, the deconvolution routine is able to determine
lifetime components considerably shorter than the IRF width. In the data shown
in Fig. 5 the fast component, t1, of the 3-ps data is split into two, with a t1
around 13 ps and a low-amplitude component, t2, of about 60 ps. The distribution
of t1 is shown in the histogram on the right of Fig. 5.
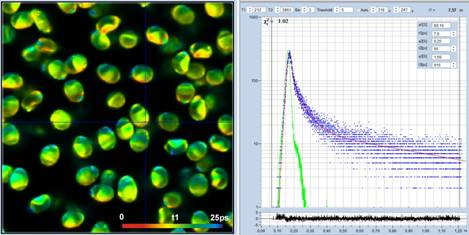
Fig. 5: Spores of Paxillus involutus, time-channel width 300 fs,
4096 time channels, observation-time interval 1.25 ns. Left: Image of the fast
decay component, t1, colour scale 0 to 25 ps. Middle: Decay curve (blue),
IRF (green), and decay parameters at the cursor position. Right: Histogram of
the t1 values over the pixels of the image.
Discussion
The results shown above demonstrate that
FLIM systems with the -NXX versions of the bh TCSPC/FLIM modules and HPM-100-06
detectors are able to resolve decay components in the sub‑10ps range.
This does not mean, however, that measurements in the ultra-fast decay domain
are straightforward. A frequent problem are reflections in the NDD beam path of
the microscope. To collect photons which are scattered on the way out of the
sample a lens projects an image of the microscope lens on the detector. Usually
even a two-step projection is used: A first lens projects an image of the
microscope lens on a second lens in front of the detector. The second lens
projects an image of the first lens on the active area of the detector. This
design has the advantage that the diameter of the beams can be kept smaller,
and that a roughly parallel part of the beam is available in which filters can
be placed. However, the principle is prone to optical reflections. Photons
reflected at the detector or another optical surface are likely to be projected
back onto another surface, reflected a second time, and fed back to the
detector some 10 or 100 ps later. The result are ugly reflections in the
decay curves. In data with ultra-fast components of high amplitude the problem
is enhanced by the high intensity ratio between the peak and the later part of
the decay curves. In the NDD light path of the DCS-120MP system the problem has
been accounted for by carefully selecting lens curvatures to avoid collimated
reflection from the lens surfaces.
Moreover, the design of the non-descanned
optics does not automatically guarantee that the optical path length is
constant for beams of all angles and over the entire aperture. The most
critical element is the lens in front of the detector. This lens has a short
focal length and a large diameter. Thus it has a large amount of spherical aberration.
That means rays entering the lens at the periphery and the centre have different
effective path length and different transit times. The differences can be on
the order of a few picoseconds. In our system the effect causes a slight
increase of the IRF width, from 18 to 19 ps fwhm in a free beam to about
23 ps in the NDD beam path. In first approximation, the broadening is the
same for the fluorescence measurement and the IRF measurement. It has thus
little influence on the results. Changes can, however, occur if the sample is
not correctly focused or if microscope lenses are changed. In these cases the
intensity distribution over the beam cross section and, consequently, the
transit time distribution change.
Another potential problem is related to IRF
measurement. There is no other way than to measure the IRF via an SHG process.
At first glance this appears to be an ideal solution. SHG is (within the resolution
of the system) infinitely fast, and it is emitted at high intensity. Possible
contamination with fluorescence therefore has little effect on the result.
Nevertheless, there is a problem. SHG is emitted in forward direction. Normally,
enough light from within the sample is scattered back into the detection beam
path. However, light leaving the sample at the back can be reflected or
scattered back, and transferred to the detector. The reflected light arrives
with a delay and thus broadens the recorded IRF or causes nasty secondary
pulses. It is therefore important to cover the back of the sample with an absorbent
cover. But even then, an unknown portion of the signal can come back from the
cover. Taking into account that the forward emission is much stronger than the
backward emission an influence on the recorded IRF shape cannot entirely be
excluded. All in all, attention to the optical details is recommended when
ultra-high resolution FLIM data are recorded.
References
1. W. Becker, The bh TCSPC Handbook, 9th ed. (2021). Available at
www.becker.hickl.com. Printed copies available from bh.
2. Becker & Hickl GmbH, The HPM‑100-40 Hybrid Detector.
Application note, available on www.becker-hickl.com
3.
W. Becker, B. Su, K. Weisshart, O. Holub, FLIM
and FCS Detection in Laser-Scanning Microscopes: Increased Efficiency by GaAsP
Hybrid Detectors. Micr. Res. Tech. 74, 804-811
(2011)
4.
Becker & Hickl GmbH, SPCImage NG next
generation FLIM data analysis software. Overview
brochure, available on www.becker-hickl.com
5. Becker & Hickl GmbH, Sub-20ps IRF Width from Hybrid Detectors
and MCP-PMTs. Application note, available on www.becker-hickl.com
6. Becker & Hickl GmbH, Two-Photon FLIM with a Femtosecond Fibre
Laser. Application note, available on www.becker-hickl.com
7. W. Becker, Bigger and Better Photons: The Road to Great FLIM
Results. Education brochure, available on www.becker-hickl.com.
8. W. Becker, C. Junghans, A. Bergmann, Two-Photon FLIM of Mushroom
Spores Reveals Ultra-Fast Decay Component. Application note (2021), available
on www.becker-hickl.com
9. W. Becker, T. Saeb-Gilani, C. Junghans, Two-Photon FLIM of Pollen
Grains Reveals Ultra-Fast Decay Component. Application note (2021), available
on www.becker-hickl.com
10. W. Becker, V. Shcheslavskiy, C. Junghans, T. Saeb-Gilani, A.
Bergmann, O. Garanina, V. Elagin, Ultra-Fast Fluorescence Decay in Biological
Material. Presentation at 19th AIM workshop, Berkeley (2022). Video available
on www.becker-hickl.com.
Contact:
Wolfgang Becker
Becker & Hickl GmbH
Berlin, Germany, https://www.becker-hickl.com
Email: becker@becker-hickl.com