We present the integration of a bh TCSPC FLIM system into an atomic force microscope (AFM). The AFM FLIM system is based on the NTEGRA SPECTRA AFM/NSOM system of NT-MDT, Moscow, and a Simple-Tau 150 TCSPC FLIM system of Becker & Hickl, Berlin. We demonstrate that the integrated system is able to record combined AFM / FLIM images of E-coli bacteria, HEK cells, and polymers.
An AFM/NSOM System with Fluorescence Lifetime Imaging
Wolfgang Becker, Vladislav Shcheslavskiy, Becker & Hickl GmbH
Abstract: We present the integration of fluorescence lifetime imaging (FLIM) into an atomic force microscope (AFM). The system is based on the NTEGRA SPECTRA AFM/NSOM system of NT-MDT, Moscow, and a Simple-Tau 150 TCSPC FLIM system of Becker & Hickl, Berlin. We demonstrate that the integrated system is able to record combined AFM / FLIM images of E-coli bacteria, HEK cells, and polymers.
Introduction
Since their broad introduction in the early 90s confocal and two-photon laser scanning microscopes have initiated a breakthrough in imaging. The applicability of multi-photon excitation, the optical sectioning capability and the superior contrast of these instruments make them an ideal choice for fluorescence imaging of biological samples.
The fluorescence of organic molecules is not only characterised by the emission spectrum, it has also a characteristic lifetime. Any energy transfer between an excited molecule and its environment in a predictable way changes the fluorescence lifetime. Since the lifetime does not depend on the concentration of the chromophore fluorescence lifetime imaging is a direct approach to all effects that involve energy transfer. Typical examples are the mapping of cell parameters such as pH, ion concentrations or oxygen saturation by fluorescence quenching, or fluorescence resonance energy transfer (FRET) between different chromophores in the cell [1].
Furthermore, combined intensity/lifetime imaging is a powerful tool to distinguish between different fluorescence markers in multi-stained samples and between different natural fluorophores of the cells themselves. These components often have ill-defined fluorescence spectra but are clearly distinguished by their fluorescence lifetime.
However, while confocal FLIM provides a significant amount of chemical information on the sample, its spatial resolution is diffraction limited. Therefore, it would be natural to combine FLIM with another high-resolution microscopy technique. One of the relatively new and established techniques for topographical imaging of both biological and inorganic samples at nanoscale resolution is atomic force microscopy (AFM). AFM has several modes of operation (contact, tapping and non-contact). One of the most developed modes of AFM for biology applications is a tapping mode: that is when the tip is oscillated at its resonance frequency, and the force applied by the tip is quite low to enable imaging of soft biological structures with ultrahigh resolution. The label-free topographical, electrical, magnetic and mechanical information that can be obtained from the AFM correlated with the fluorescence lifetime map provides an important tool for deeper investigation of a sample properties.
System description
AFM / NSOM System
A photo of the NTEGRA SPECTRA system is shown in Fig. 1, left, the sample excitation principle in Fig. 1, right.

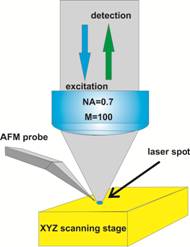
Fig. 1: Left: Photo of NTEGRA SPECTRA system. Right: Operation principle of the AFM head with built-in objective lens
The system works in the AFM tapping mode. The fluorescence is collected back though the microscope lens and projected into a confocal pinhole. Light passing the pinhole is spectrally filtered by a monochromator in the NTEGRA optics, and projected on the FLIM detector. The configuration used in the NTEGRA system and a special AFM probe shape have the advantage that it can be used also for opaque samples. Moreover, it can be used to perform beam scanning in addition to sample scanning, which is a welcome feature for co-localisation measurements and for measurements with enhanced Raman scattering [8].
For AFM and FLIM imaging, the sample is placed on a three-axis piezo stage (Fig. 1, right). The piezo stage is used both for keeping the AFM probe at a constant distance to the sample surface, and for raster scanning in x and y. The piezo stage has a scan area of 120µm x 120µm in X and Y, and a scan range of 10µm in Z. All the images presented here were collected with a tapping mode AFM silicon cantilever coated with TiN. The length of the cantilever was 125µm. The probe had a force constant of 5.1N/m, compatible with imaging of biological objects, and a resonant frequency of 160 kHz.
FLIM System
For fluorescence lifetime imaging, the system was upgraded with a bh BDL‑SMN 488 nm picosecond diode laser [2], a bh Simple-Tau 150 TCSPC FLIM system [1], and a bh HPM‑100-40 GaAsP hybrid detector [1, 3]. The system components are shown in Fig. 2.



Fig. 2: Components of the FLIM system. Left to right: BDL‑SMN ps diode laser, Simple-Tau 150 TCSPC FLIM system, HPM‑100-40 hybrid detector.
The BDL‑SMC laser generates pulses of 488 nm wavelength, 50 ps pulse duration, and 80, 50, or 20 MHz repetition rate. The laser has a beam-profile corrector and a Qioptiq single-mode fibre connector. The power injected into the fibre is about 2 mW at 80 MHz. Higher power is available if an increase of the pulse duration is acceptable [2].
The Simple-Tau 150 consists of a laptop computer with a bus extension cable and an electronics box. The box contains a bh SPC‑150 TCSPC/FLIM module and a DCC‑100 detector controller. The HPM‑100 detector contains a Hamamatsu R10467-40 hybrid PMT tube, the high-voltage generators for the operating voltages of the tube, and a GHz-bandwidth low-noise preamplifier. The detector has a detection efficiency of about 50% and is free of afterpulsing. The result is a very good photon efficiency of the FLIM measurement: The number of photons required to reach a given lifetime accuracy is very close to the ideal value [1].
Fluorescence lifetime imaging in the SPC‑150 TCSPC card is based on a multi-dimensional TCSPC process [1]. The principle is shown in Fig. 3, left, the general system architecture in Fig. 3, right. The TCSPC module receives the single-photon pulses from the detector, the timing reference pulses from the laser, and the scan synchronisation pulses (start-of-frame, start-of-line, start-of-pixel) from the scan controller of the NTEGRA system. For every photon, the TCSPC system determines the time, t, in the laser pulse period, and the location (x, y, of the AFM tip) in the scan area. From these pieces of information, the TCSPC process builds up a photon distribution over x, y, and t. The distribution can be interpreted as an array of pixels over x and y, each containing a full fluorescence decay function over the time in the pulse period, t. The advantage of the process is that it records a full fluorescence decay function in each pixel, not only a fluorescence lifetime. Moreover, it records at near-ideal photon efficiency, and it can be used at any scan rate. In laser scanning microscopes (with scan rates on the order of 106 pixels per second) the acquisition is run over a larger number of frames. At the slow scan rates used in AFM / NSOM the data are usually acquired over a single frame. The result is independent of the scan rate if the total acquisition time and the detection rate are the same [1].
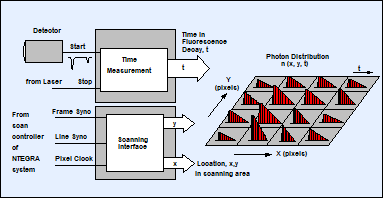
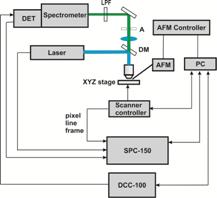
Fig. 3: Left: Principle of TCSPC FLIM. Right: Schematic diagram of the AFM-FLIM setup. LPF: long-pass filter (495LP), DM: dichroic mirror 510lpxru, DET: HPM-100-40 hybrid detector.
The SPC‑150 module in combination with bh 64 bit SPCM data acquisition software is able to record images of megapixel size [1, 4]. This makes the FLIM system compatible with the high pixel numbers frequently used in AFM. Frames sizes of 2048 x 2048 pixels, 1024 x 1024 pixels, and 512 x 512 pixels are possible at a time resolution of 256, 1024, and 4096 time channels per pixel, respectively [1]. The high number of time channels can be useful when NSOM decay data contain both very fast and very slow decay components.
All data presented here were recorded by the procedure described above, and in the FIFO Imaging mode of the SPCM data acquisition software. FLIM data analysis was performed by bh SPCImage FLIM data processing software [1]. FLIM data were combined with AFM topography data by using NOVA software of NT-MDT.
Sample Preparation
The preparation of the samples for AFM is essentially the same as for normal FLIM measurements, apart from the fact that the samples should not be covered with a cover slip that would prevent access of the AFM probe to the surface of the sample.
Results
E-coli bacteria transfected with EGFP and phiYFPv
E-coli bacteria were transfected with EGFP and phiYFPv. The cell suspension was deposited on the glass slide and dried. Both height and phase (not shown) images were acquired and further processed with NOVA software. Fig. 4, A, shows a topography image (512x512pixels) of a relatively dense population of e-coli bacterial cells. The overall scanning range is 40x40µm. Fig. 4, B to D shows a 4x4 µm area in the same data. Fig. 4, C, is the FLIM image, Fig. 4, D, is an overlay of the fluorescence lifetime with the topography data. A fluorescence decay function in a 5x5 pixel area of Fig. 4, C, is shown in Fig. 5.
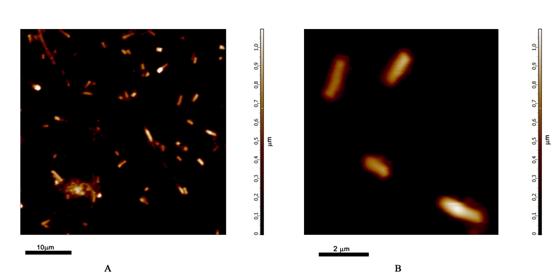
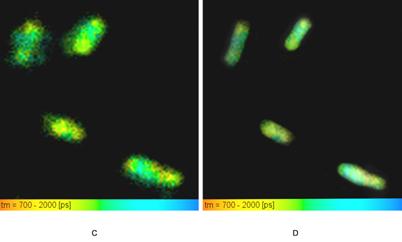
Fig. 4: E-coli bacteria transfected with EGFP and phiYFPv. A: AFM (topography) data, scan area 40x40 µm. B: AFM (topography) data, scan area 4x4 µm. C: NSOM FLIM, colour = fluorescence lifetime, brightness = fluorescence intensity, scan area 4x4 µm. D: AFM / FLIM, colour = fluorescence lifetime, height of topography = brightness, scan area 4x4 µm.

Fig. 5: Fluorescence decay function in a 5x5 pixel area of the lower right cell in Fig. 4, C.
HEK cell transfected with GFP
Fig. 6 shows data recorded at a HEK (human embryonic kidney) cell. Left to right, it shows the AFM topography image, the lifetime images, and a combined lifetime-topography image.
![]()
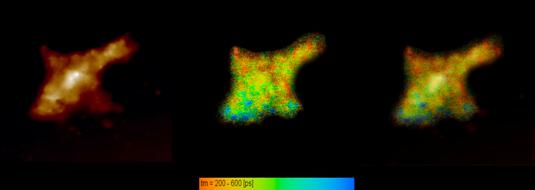
Fig. 6: HEK (human embryonic kidney) cell. Left: Topography. Middle: Lifetime, red to blue represents 200-600 ps. Right: Lifetime (colour) combined with topography (brightness)
Polymer
Fig. 7, left to right, shows a topography image, a lifetime image, and the combined topography-lifetime image of a polymer blend. Fig. 8 shows the fluorescence decay function in a 3x3 pixel area of the bright feature on the lower right of Fig. 7.
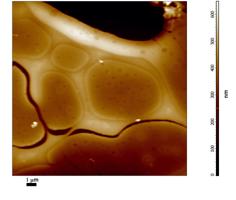
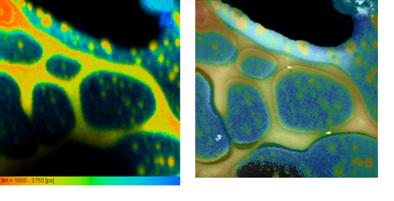
Fig. 7: NSOM FLIM of a polymer blend. Left to right: Topography, lifetime image, Lifetime combined with topography.
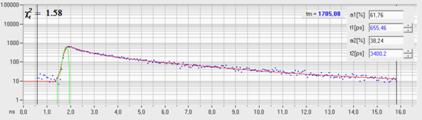
Fig. 8: Fluorescence decay function in a 3x3 pixel area of the bright feature on the lower right of Fig. 7.
Summary
AFM/NSOM systems can easily be combined with bh FLIM systems if they provide an output for the synchroninisation pulses, new-frame, new-line, new-pixel from the piezo controller, an optical input from a pulsed laser, and an optical output to a photon counting detector. These requirements are met by the NT-MDT NTEGRA system. We tested a combination of the NTEGRA with a bh Simple-Tau 150 TCSPC FLIM system, a bh BDL-SMN ps diode laser, and a bh PMH-100-40 hybrid detector. The advantage of AFM FLIM is that both high-resolution topography data and fluorescence lifetime data are obtained. The data can be combined into images that show the topography as brightness and the fluorescence lifetime as colour. Results were presented for E Coli cells, HEK cells, and a polymer blend. The technique described can be extended to combined fluorescence/phosphorescence lifetime imaging [5], laser wavelength multiplexing [1], multi-wavelength detection [1, 6], and NIR detection [7].
References
1. W. Becker, The bh TCSPC handbook. Becker & Hickl GmbH, 6th Edition (2015), www.becker-hickl.com, printed copies available from bh
2. Becker & Hickl GmbH, BDL-SMN series picosecond diode lasers. User handbook, www.becker-hickl.com
3. Becker, W., Su, B., Weisshart, K. & Holub, O. (2011) FLIM and FCS Detection in Laser-Scanning Microscopes: Increased Efficiency by GaAsP Hybrid Detectors. Micr. Res. Tech. 74, 804-811
4. Studier, H., Becker, W. Megapixel FLIM. Proc. SPIE 8948, (2014)
5. Becker, W., Su, B., Bergmann, A., Weisshart, K. & Holub, O. (2011) Simultaneous Fluorescence and Phosphorescence Lifetime Imaging. Proc. SPIE 7903, 790320
6. Becker & Hickl GmbH, MWFLIM GaAsP multi-spectral FLIM detectors. Data sheet, www.becker-hickl.com
Contact: Wolfgang Becker, becker@becker-hickl.com

