SPCM Software Runs Online FLIM at 10 Images per Second
W. Becker, C. Junghans, Becker & Hickl GmbH
Abstract: Version 9.72 SPCM software of the bh TCSPC/FLIM systems displays fluorescence
lifetime images at a rate of 10 images per second. The calculation of the
lifetime images is based on the first moment of the decay data in the pixels of
the images. The first-moment technique combines short calculation times with
near-ideal photon efficiency. It works for all SPC-150, SPC-150N, SPC-160, and
SPC-830 FLIM systems that use fast scanning. In combination with the preview
mode of the SPCM software it can be used to select interesting cells within a
larger sample for subsequent high-accuracy FLIM acquisition. In FLIM
experiments with longer acquisition time it helps the user evaluate the
signal-to-noise ratio of the data and decide whether enough photons have been
recorded to reveal the expected lifetime effects in the sample.
Fast FLIM
Fast FLIM by TCSPC has been demonstrated on
several occasions. Time-series FLIM by a record-and-save procedure has been
described in [2], time-series FLIM by memory swapping in [3] and [11], temporal
mosaic FLIM in [3] and [6], and triggered accumulation of time series for
imaging Ca++ transients in [3, 6, 7] and [9]. These techniques are
aiming at the recording of fast dynamic effects in the fluorescence
decay parameters, not at a fast sequential online display of lifetime
images. Online display not only requires that the FLIM data are recorded within
an extremely short acquisition time but also that the fluorescence lifetime is
calculated from the decay data in a similarly short period of time. The task is
complicated by the fact that the signal-to-noise ratio of a FLIM recording
cannot be higher than
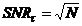 (1)
(1)
where N is the number of photons in the
pixels. The number of photons available within a given period of time (the
photon count rate) is limited by the sample itself: Excitation power or
fluorophore concentration above a certain level cause invasive effects to the
sample. Count rates obtained in FLIM experiments are thus rarely higher than a
few MHz. The speed of online-FLIM is therefore limited by the decrease of the
signal-to-noise ratio with increasing image rate. The requirements for fast online
FLIM are therefore:
- The recording technique must record the photons in a way that the
SNR comes close to the ideal value. That means the decay curve must be recorded
in a sufficiently large number of time channels, with a negligible IRF width, and
with negligible background from detector dark counts, detector afterpulsing, or
daylight pickup [12]. TCSPC comes very close to the ideal conditions, and
achieves a signal-to-noise ratio very close to the ideal value.
- the calculation algorithm for the lifetime must not only be fast
enough to analyse the images within a time shorter than the image period but
also extract the lifetime from the recorded data at the ideal signal-to-noise
ratio inherent to the TCSPC data.
Fast Calculation of Lifetimes from TCSPC Data
Lifetime Calculation Algorithms
There is a number of fast algorithms for
lifetime calculation from TCSPC data. The lifetime can be derived from the
photon numbers in two time intervals (time gates), from the time until the
integral of the decay curve reaches 1-1/e of the maximum, or from the phase and
the amplitude at the fundamental frequency after transformation in the
frequency domain. All these algorithms have their own problems: Time-interval
analysis delivers systematic errors if the rise of the fluorescence does not
coincide with the beginning of the first time interval, or a sub-optimal SNR if
the fluorescence rises before the first time interval. Moreover, the width of
the time gates must be adjusted to the expected lifetime, which is possible
only if the approximate lifetime is known. The lifetime determination via the
integral does not use all photons, and is influenced by the uncertainty of the
1-1/e point of the integral. The SNR is thus sub-optimal. The SNR of frequency
domain analysis is sub-optimal if only the phase and amplitude at the
fundamental frequency are considered (the photons are weighted differently depending
on their time in the decay curve). Sub-optimal SNR is, however, compensated by
the ability to automatically combine pixels of similar phase/amplitude
signature. The result is a phasor plot, not a lifetime image. It therefore
does not immediately match the requirements of online FLIM.
First-Moment Algorithm
An almost ideal SNR from TCSPC data is
obtained by calculating the lifetime via the first moment of the decay data [8].
The method has been suggested first by Z. Bay in 1950 for the determination of
excited nuclear state lifetimes in coincidence experiments [1]. The first
moment of a photon distribution is
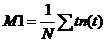 (2)
(2)
with N = total number of
photons, t = time of individual time channels, n(t) = photon
number in individual time channels
The first moment can also be considered the
average arrival time of all photons in the decay curve. For a
single-exponential decay, it can easily be shown that the first moment delivers
an ideal SNR: The standard deviation of the photon arrival time is t, and the
standard deviation of the average arrival time of a large number of photons is 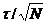 . The
relative standard deviation, or the SNR, is thus
. The
relative standard deviation, or the SNR, is thus 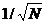 , and the signal-to-noise ratio
is
, and the signal-to-noise ratio
is 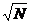 .
.
The fluorescence lifetime (of a
single-exponential decay approximation) is the difference of the first moment
of the fluorescence and the first moment of the IRF:
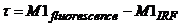 (3)
(3)
The relations are illustrated graphically
in Fig. 1. The blue dots are the photon numbers in the time channels of the
pixel, the green curve is the IRF. M1 of Fluorescence is the first moment
calculated according to (2), M1 of IRF is the M1 of the IRF calculated by the
same formula. Tau is the fluorescence lifetime calculated according to (3).
The red curve is the convolution of a single-exponential function, f(t) = e-t/t, with the
IRF.
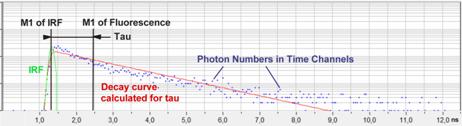
Fig. 1: First-moment calculation of fluorescence lifetime. The lifetime is
the difference of the first moment of the fluorescence and the first moment of
the IRF.
For a double-exponential decay, it can be
shown that (3) delivers the intensity-weighted average of the lifetimes of the
two components. The signal-to-noise ratio remains very close to 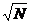 for a wide
range of lifetime and intensity ratios of the components.
for a wide
range of lifetime and intensity ratios of the components.
To obtain correct lifetimes the first moment
of the IRF has to be known. This is no problem for TCSPC FLIM. M1IRF
can be obtained either by analysing a FLIM data file with SPCImage [3], or from
decay data of a fluorophore with known lifetime. In any case, the IRF can be
recorded with a high N, its uncertainty therefore does not significantly
contribute to the uncertainty of t.
Other requirements for the M1 calculation
of lifetimes are that the background of the decay signal is negligible, and
that the entire decay curve is recorded up to a time channel beyond which no
more photons are recorded. Also this is no problem for fast online display. Due
to the short acquisition time, background is low or even not detectable, and
the decay function drops quickly to the point where later photons are unlikely
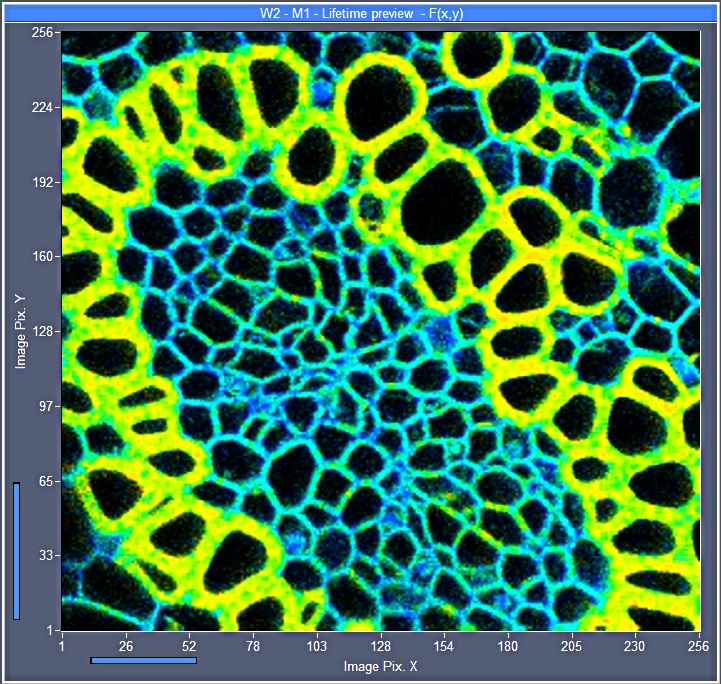
to be detected. Examples can be seen in Fig. 2 and Fig. 3, middle.
Binning of Decay Data
Online FLIM at image rates faster than one image
per second delivers very low photon numbers in the pixels. An example is shown
in Fig. 2. The decay curve (shown left) is from a single pixel of a FLIM
recording of 256x256 pixels, recorded at an acquisition time of 0.2 seconds (5
frames per second). The entire curve contains about 45 photons. The
corresponding FLIM image is shown in Fig. 2, right. According to (1) the SNR of
the fluorescence lifetime in the bright pixels of the image is about 7. The
noise in the lifetime can easily be seen in Fig. 2.
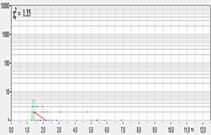
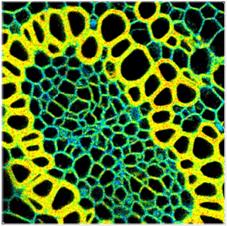
Fig. 2: Online FLIM with acquisition time 0.2 seconds (5 frames/second).
256x256 pixels, no binning. Left: Decay curve in brightest pixel, the photons
are barely visible between t = 1ns ... 2ns. Right: Lifetime
image calculated by M1 algorithm, red to blue = 1000 to 3000 ps.
A substantial improvement is obtained by binning the decay data for
the lifetime analysis. The binning algorithm used in SPCM is the same as in
SPCImage. For every pixel of the image, it uses not only the photons in this
pixel but also the photons in the pixels around, see Fig. 3, left. On average,
this yields a photon number 9 times larger than without binning (Fig. 3,
middle), and a 3 times better SNR of the lifetime. The lifetime image is built
up from the intensity data of the unbinned pixels and the lifetime data from
the binned pixels. The image calculated this way is shown in Fig. 3, right.

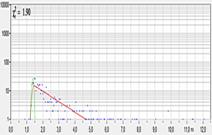
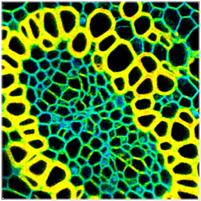
Fig. 3: Online FLIM, parameters same as Fig. 2, but with binning of
lifetime data, 3x3 pixels. Left: Decay curve in brightest pixel. Right:
Lifetime image calculated by M1 algorithm, red to blue = 1000 to 3000 ps.
The improvement in image quality is
striking: The noise in the lifetime is much lower, but, surprisingly, there is
no apparent reduction in image definition. This has two reasons. The first one
is the way the human eye-brain combination processes images. Perception of
image definition comes exclusively from the intensity, the colour is just an overlay
on the intensity image. Since the intensity data have not been binned the apparent
definition is not impaired by binning. The second one is the way images are
recorded in microscopy. Usually, the images are oversampled, i.e. the Airy disc
(or point-spread function) is imaged on an area of several pixels. Binning has,
of course, little effect on data which are already smoothed by spatial convolution
with the Airy disc.
Implementation in the SPCM Software
The Online-Lifetime display function is
implemented in the SPCM software, version 9.72 or later. To activate the online
display, open the 3D Trace Parameter panel and define a display window for
Lifetime Preview data, see Fig. 4, left.
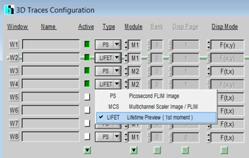
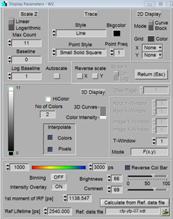
Fig. 4: SPCM Definitions for online lifetime display
The pseudo-colour range for the lifetime
display is defined in the Display Parameter panel corresponding to the selected
display window. The display parameters also have sliders for brightness and
contrast. The sliders work independently of the scale definitions in the upper
part of the display parameters, they are also available when autoscale is
selected.
As described above, the first-moment
calculation needs the first moment of the IRF. This can either be typed in or
calculated from a FLIM data file. In the first case, the moment of the IRF can
be taken from the SPCImage data analysis software. Load a FLIM file into
SPCImage, select Model, 1st Moment, and find the 1st Moment of IRF in
the lower right part of the SPCImage panel. The file can be from any sample. It
is only important that it was recorded by the same FLIM system, with similar
optical and electrical path length, and with similar TAC parameters as the data
to be displayed.
In the second case SPCM uses a FLIM data
file from a reference sample. This sample should have a uniform lifetime, and
the lifetime must be known or determined with SPCImage. The file must, of
course, be recorded with the same instrument configuration, and with the same
TAC parameters that are used for the online display. For diode-laser
excitation, it is recommended to use also the same (electrical) laser power. If
you need different laser power, change the power optically [3, 4]. It is not
necessary to record a new reference file every day. The bh TCSPC modules have
an extremely good timing stability [3]. The reference data therefore remain valid
over weeks or months.
To use the online lifetime display for FLIM
at high image rate the Repeat function of the SPCM software in combination
with a short Collection Time and a short Repeat Time is used. For frequent
updates of the FLIM display during a measurement with long acquisition time the
desired update interval is defined by Display Time. The parameters are
accessible in the lower left part of the SPCM main window. For details please
see The bh TCSPC Handbook [3] or handbooks of the DCS-120 system [4] or of the
FLIM systems for the Zeiss laser scanning microscopes [5].
Typical Results
Online lifetime images recorded at a rate
up to 10 images per second (acquisition time 100 ms) are shown in Fig. 5
through Fig. 7. The images were recorded by a bh DCS-120 confocal scanning
system [4]. A convallaria sample was used as a test object, the excitation
wavelength was 488 nm. The count rates were in the range of 1 to 2 MHz,
averaged over the entire image. The data were recorded with 256 time channels
and a time channel width of 49 ps. All images are displayed with the same
lifetime scale, 1000 ps (red) to 3000 ps (blue).
Fig. 5 shows 128x128-pixel images recorded
with acquisition times of 0.1 s, 0.5 s, and 2 s. The upper row shows images
without binning, the lower row images with 3x3 pixel binning of the decay data.
The images show that lifetime images of reasonable quality are obtained even at
an acquisition time of 100 ms, or an image rate of 10 per second.
Good images at a resolution of 256x256
pixels and 512x512 pixels were obtained at about 5 images and 2 images per
second, respectively (Fig. 6 and Fig. 7). Also here, binning reduces the
lifetime noise substantially without causing noticeable blur of the images.
With 64-bit SPCM software, the image size can
be increased to 1024x1024 pixels and more [3, 4, 14]. The M1 algorithm is fast
enough to process such data in a fraction of a second. Images of reasonable
lifetime resolution are obtained down to an acquisition time of 2 seconds, or
an image rate of 0.5 per second. An example is shown in Fig. 8
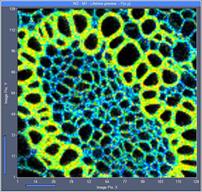
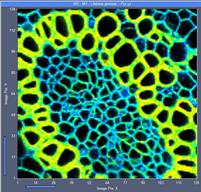
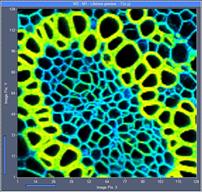
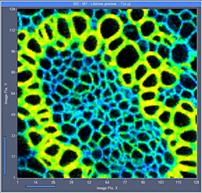
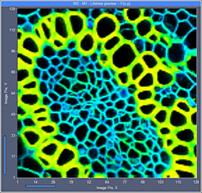
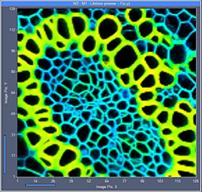
Fig. 5: 128x128-pixel images. Acquisition time 0.1s, 0.5s, 2s. Upper row:
No binning. Lower row: Binning 3x3 pixels
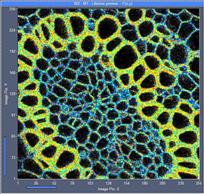
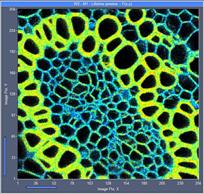
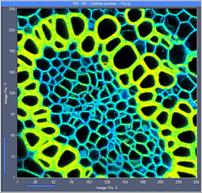
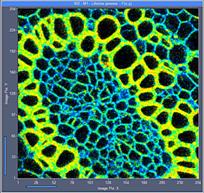
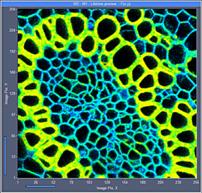
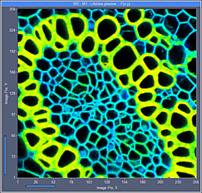
Fig. 6: 256x256-pixel images. Acquisition time 0.2s, 0.5s, 2s. Upper row:
No binning. Lower row: Binning 3x3 pixels
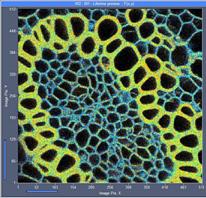
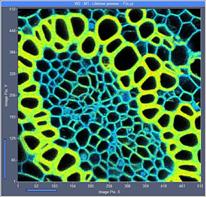
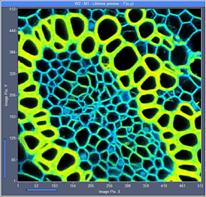
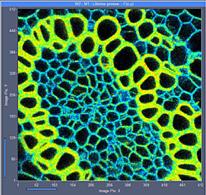
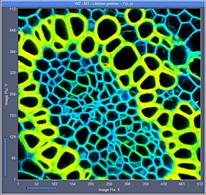
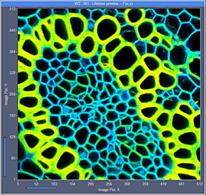
Fig. 7: 512x512-pixel images. Acquisition time 0.5s, 2s, 5s. Upper row: No
binning. Lower row: Binning 3x3 pixels
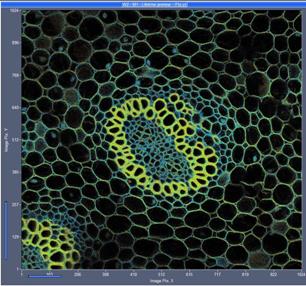
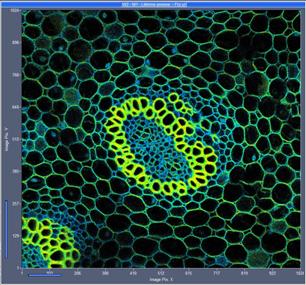
Fig. 8: 1024x1024-pixel image, acquisition time 2 seconds. Left without,
right with 3x3 pixel binning of the decay data.
Discussion
The results shown above demonstrate that fast
online FLIM is feasible almost up to the maximum frame rate of the commonly
used galvanometer scanners. It should be taken into account, however, that the
examples shown here were recorded with a convallaria sample. In this sample,
the lifetime varies over a range of about 1:2.5. In real-world FLIM samples the
lifetime variation is often smaller. Selecting FRET-positive cells in a culture
of donor-and-acceptor expressing cells requires a lifetime resolution of about
1:1.25. Obtaining images of similar quality as for the convallaria would require
four times the photon number, and thus four times the acquisition time, see
(1). This would be about 0.4 seconds for a 128x128 pixel image with binning. This
is still an acceptable image rate. Even in clinical FLIM applications, with
metabolism-induced lifetime changes on the order of 1:1.1 [10, 13] images can
probably be displayed at rates faster than one image per second.
References
1. Z. Bay, Calculation of decay times from coincidence experiments. Phys.
Rev. 77, 419 (1950)
2. W. Becker, Advanced time-correlated single-photon counting techniques. Springer,
Berlin, Heidelberg, New York, 2005
3. W. Becker, The bh TCSPC handbook. 6th edition, Becker & Hickl
GmbH (2015), available on www.becker-hickl.com
4. Becker & Hickl GmbH, DCS-120 Confocal Scanning FLIM Systems, 6th
ed. (2015), user handbook. www.becker-hickl.com
5. Becker & Hickl GmbH, Modular FLIM systems for Zeiss
LSM 710 / 780 / 880 family laser scanning microscopes. 6th ed. (2015),
user handbook. available on www.becker-hickl.com
6.
W. Becker, V. Shcheslavskiy, H. Studier, TCSPC FLIM with Different
Optical Scanning Techniques, in W. Becker (ed.) Advanced time-correlated single
photon counting applications. Springer, Berlin, Heidelberg, New York (2015)
7.
W. Becker, V. Shcheslavkiy, S. Frere, I. Slutsky,
Spatially Resolved Recording of Transient Fluorescence-Lifetime Effects by
Line-Scanning TCSPC. Microsc. Res. Techn. 77, 216-224 (2014)
8. I. Isenberg, R.D. Dyson, The analysis of fluorescence decay by a
method of moments. Biophys. J. 9, 1337-1350 (1969)
9.
S. Frere, I. Slutsky,
Calcium imaging using Transient Fluorescence-Lifetime Imaging
by Line-Scanning TCSPC. In: W. Becker (ed.) Advanced time-correlated single
photon counting applications. Springer, Berlin, Heidelberg, New York (2015)
10. S. Kalinina, V. Shcheslavskiy, W. Becker, J. Breymayer, P. Schäfer,
A. Rück, Correlative NAD(P)H-FLIM and oxygen sensing-PLIM for metabolic mapping.
J. Biophotonics (2016)
11. V. Katsoulidou, A. Bergmann, W. Becker, How fast can TCSPC FLIM be
made? Proc. SPIE 6771, 67710B-1 to 67710B-7 (2007)
12. M. Köllner, J. Wolfrum, How many photons are necessary for
fluorescence-lifetime measurements?, Phys. Chem. Lett. 200, 199-204 (1992)
13. W. Y. Sanchez, M. Pastore, I. Haridass, K. König, W. Becker, M. S.
Roberts, Fluorescence Lifetime Imaging of the Skin. In: W.
Becker (ed.) Advanced time-correlated single photon counting applications.
Springer, Berlin, Heidelberg, New York (2015)
14. H.
Studier, W. Becker, Megapixel FLIM. Proc. SPIE 8948 (2014)
Contact:
Wolfgang Becker
Becker & Hickl GmbH
Berlin, Germany
Email: becker@becker-hickl.com