Principles
Table of Contents:
- Introduction to Stimulated-Emission Depletion (STED) Microscopy
- How STED Microscopy Achieves Optical Super-Resolution
- Combining STED Microscopy with FLIM
- Limitations of STED Microscopy in Molecular Applications
- Potential Benefits of Using STED FLIM with Medium-QE Fluorophores
- Example of STED FLIM Image Recorded with bh SPC-150 Modules and Abberior STED Microscope
Introduction to Stimulated-Emission Depletion (STED) Microscopy
Stimulated-Emission Depletion (STED) microscopy exploits the nonlinearity of stimulated emission to obtain optical super-resolution (Hell & Wichmann, 1994). The fluorescence excited by the excitation laser beam in a scanning microscope is depleted by stimulated emission induced by a second (STED) laser beam.
How STED Microscopy Achieves Optical Super-Resolution
The wavefront of the STED beam is manipulated to obtain a diffraction pattern that either has doughnut shape laying in the x-y plane or a dumbbell shape oriented along the z axis. Fluorescence remains un-depleted in the centre part of the doughnut or the dumbbell. Because stimulated emission is highly nonlinear the un-depleted volume can be made considerably smaller than the point-spread function of the excitation beam. By scanning both beams together images with optical super-resolution are obtained.
Combining STED Microscopy with FLIM
Because STED is using a scanning procedure and pulsed excitation the combination with FLIM does not pose a major problem (Auksorius et al. (2008)). The fact that the early part of the decay function is distorted by incomplete depletion can by accounted for by excluding this part from the lifetime analysis.
Limitations of STED Microscopy in Molecular Applications
Nevertheless, publications on molecular FLIM applications of STED are sparse. The fluorescence lifetime is mainly used as a discrimination parameter for different fluorophores. The reason is probably that STED traditionally used fluorophores of maximum quantum efficiency and high photostability. These fluorophores have rigid molecular structure, and thus do not exhibit noticiable lifetime changes depending on the molecular environment. A potential problem in molecular imaging applications are also additional absorbers in the cells. These absorbers can be hit by the depletion laser. Due to the high power they photobleach quickly, which either directly impairs the viability of the cells or, at least, causes oxydative stress. Examples are FRET imaging, where the STED laser destroys the acceptor, or NAD(P)H FLIM, where it destroys the FAD. Nevertheless, the may be useful applications if only STED FLIM would be used with fluorophores of medium quantum efficiency.
Potential Benefits of Using STED FLIM with Medium-QE Fluorophores
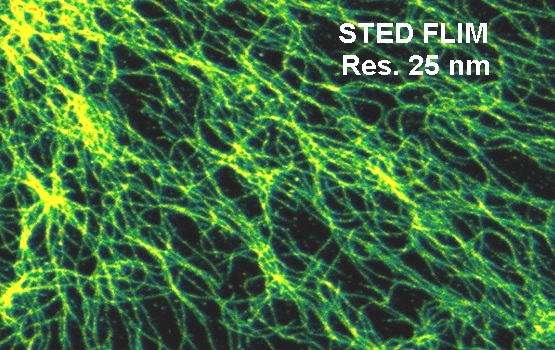
A STED FLIM image recorded with a bh SPC-150 modules on an Abberior STED microscope (Abberior Systems, Göttingen, Germany) is shown in the figure below.

Example of STED FLIM Image Recorded with bh SPC-150 Modules and Abberior STED Microscope
A fluorescence decay extracted from the data shown above is shown in the next figure. The shape of the decay is typical for STED. There is the initial peak of un-depleted fluorescence from the entire airy disc of the excitation laser, followed by the fluorescence decay function from the molecules in the ‘hole’ of the depletion disc. The correct fluorescence decay parameters are obtained by fitting only the part where the depletion is complete. Moreover, the contrast and resolution can be improved by using only fluorescence from the depleted part of the decay function. Both can be achieved by standard functions of bh SPCImage FLIM analysis software.
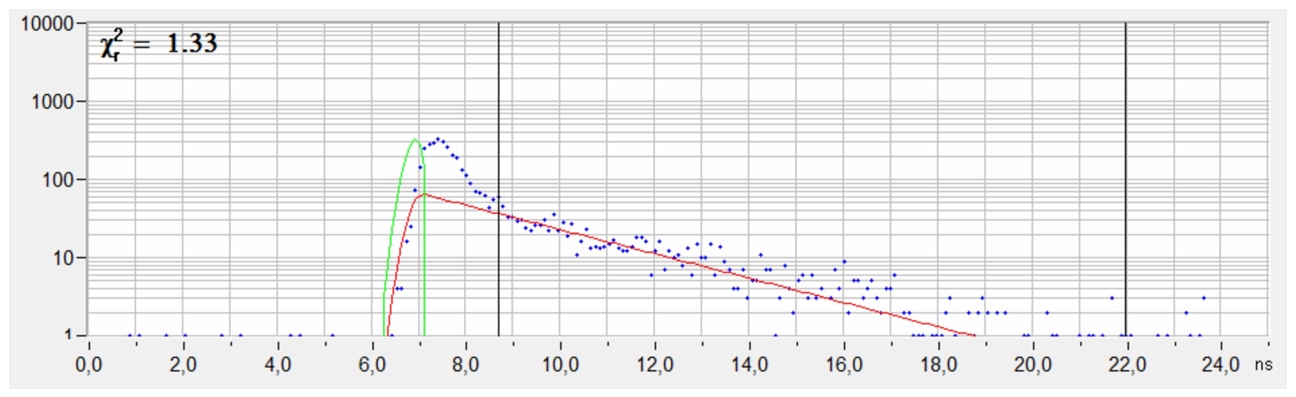
Decay curve from a selected spot of the STED FLIM image. The fluorescence intensity and the fluorescence decay time were derived from the time interval after the peak of un-depleted fluorescence.
References
- W. Hell, J. Wichmann, Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, 780-782 (1994)
- Auksorius, B. R. Boruah, C. Dunsby, P. M. P. Lanigan, G. Kennedy, M. A. A. Neil, P. M. W. French, Stimulated emission depletion microscopy with a supercontinuum source and fluorescence lifetime imaging. Opt. Lett. 33, 113-115 (2008)
- Bückers, D. Wildanger, G. Vicidomini, L. Kastrup, S.W. Hell, Simultaneous multi-lifetime multi-colour STED imaging for colocalization anlysis. Opt. Expr. 19, 3130-3143 (2011)
- D. Lesoine, S. Bose, J. W. Petrich, E. A. Smith,Supercontinuum Stimulated Emission Depletion Fluorescence Lifetime Imaging. J. Phy. Chem. B, 116, 7821-7826 (2012)
