Principles
Simultaneous NAD(P)H and pO2 Imaging
The fluorescence decay function of NAD(P)H is an indicator of whether a cell is running an oxydative (oxydative phosphorylation) or a reductive metabolism (glycolysis). The source of the change is a shift in the ratio of bound and unbound NAD(P)H. The bound and the unbound fractions have different fluorescence lifetimes, which, in turn, causes a change in the decay profile, see figure below.
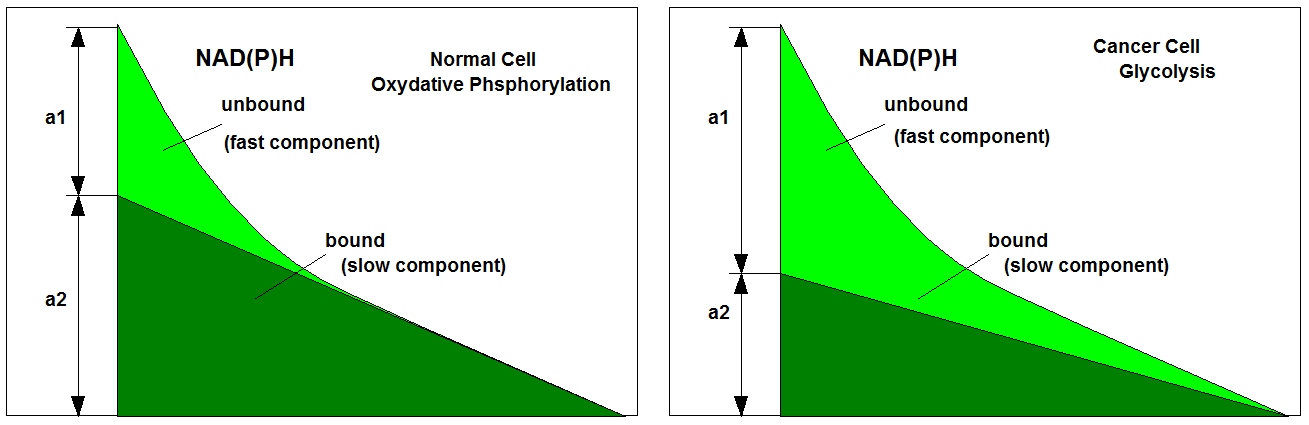
In healthy cells the metabolism is mainly oxydative, in a cancer cell it is reductive. The shape of the decay curve, in particular the a1/a2 ratio, is therefore a discrimination parameter between normal cells and cancer cells. The changes in the decay curves are expoited in Metabolic Imaging. Unfortunately, the metabolic state shifts also with the oxygen supply. Under hypoxic conditions a normal cell may shift its metabolism to glycolysis and behaves similar as a cancer cell. It is therefore desirable to have a technique available that tracks the oxygen concentration (or the oxygen partial pressure, pO2) during NAD(P)H FLIM.
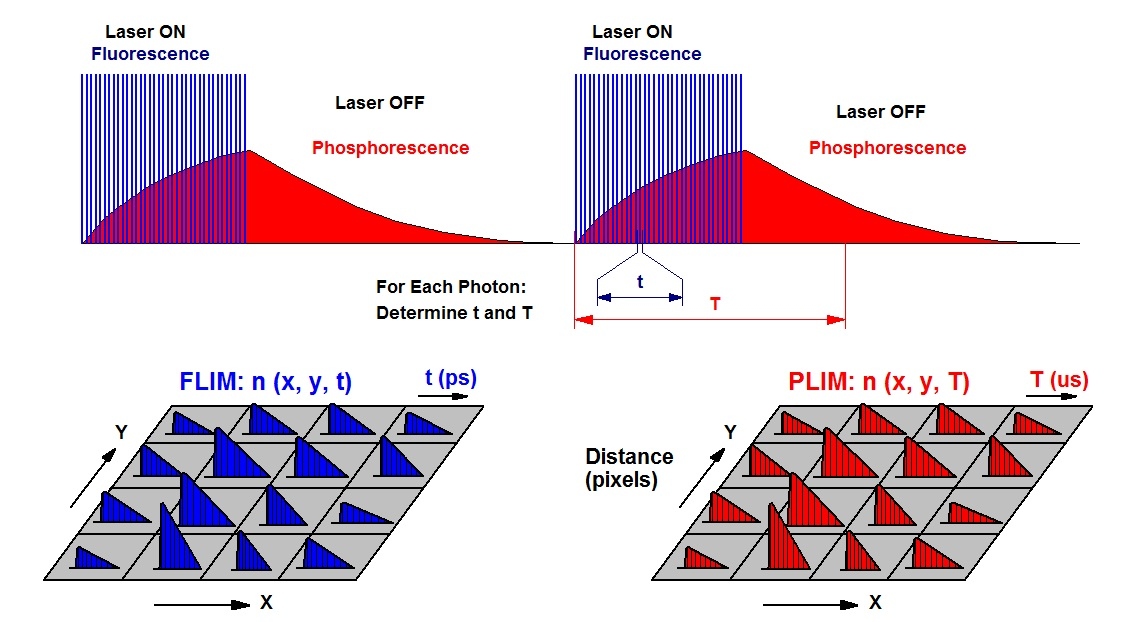
The task can be solved by using phosphorescence as a pO2 sensing function. bh’s simultaneous FLIM / PLIM technique is then used to simultaneously record the NAD(P)H fluorescence and the phosphorescence of the oxygen sensor. The technique is based on an additional modulation of the excitation pulse sequence, and building up two photon distributions. The FLIM distribution is derived from the photon times in the excitation pulse period, the PLIM distribution from the times in the modulation period. The principle is illustrated in the figure below.
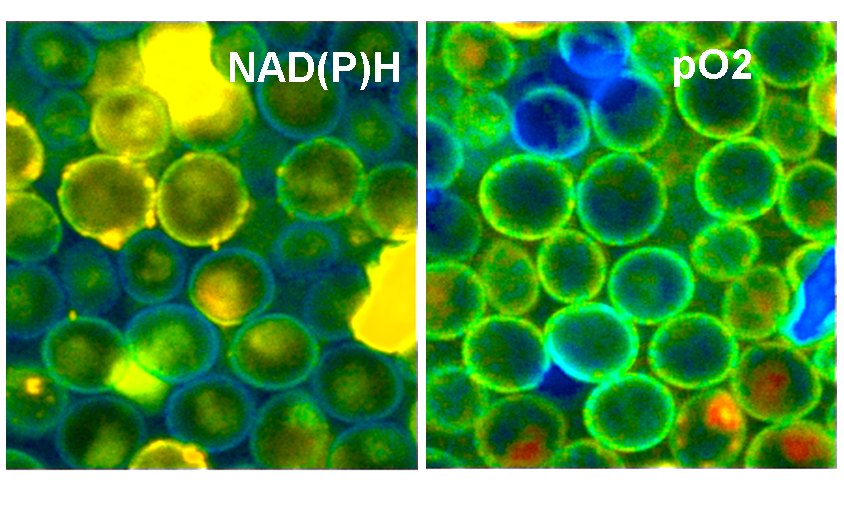
A typical result is shown in the next image. Yeast cells were stained with a ruthenium-based phosphorescence dye. The cells were then imaged by the FLIM / PLIM process described above.
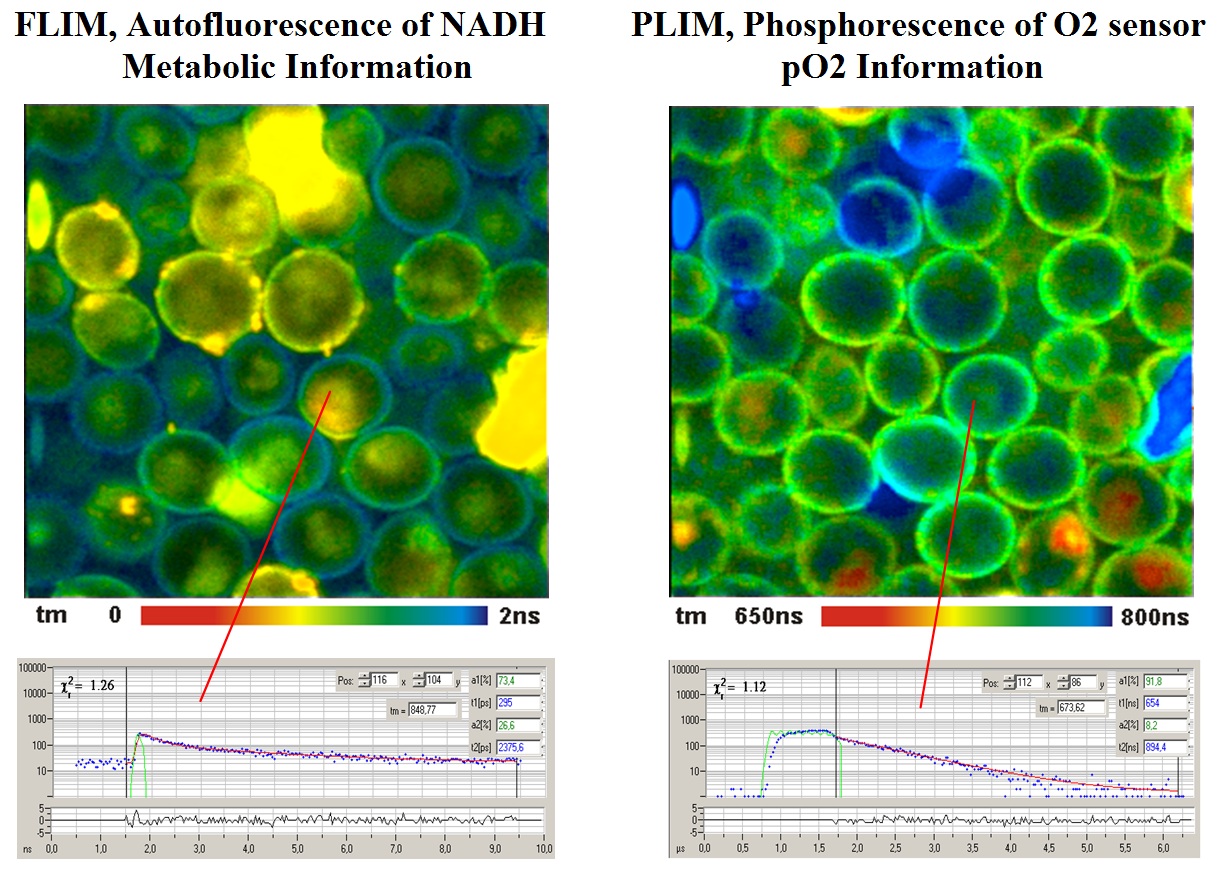
For details please see bh TCSPC handbook, chapter ‘Oxygen sensing by phosphorescence lifetime measurement’.
References
Publications related to Simultaneous FLIM/PLIM
- Baggaley, S. W. Botchway, J. W. Haycock, H. Morris, I. V. Sazanovich, J. A. G. Williams, J. A. Weinstein, Long-lived metal complexes open up microsecond lifetime imaging microscopy under multiphoton excitation: from FLIM to PLIM and beyond. Chem. Sci. 5, 879-886 (2014)
- Baggaley, M. R. Gill, N. H. Green, D. Turton, I. V. Sazanovich, S. W. Botchway, C. Smythe, J. W. Haycock, J. A. Weinstein, J. A. Thomas, Dinuclear Ruthenium(II) Complexes as Two-Photon, Time-Resolved Emission Microscopy Probes for Cellular DNA. Angew. Chem. Int. Ed. Engl. 53, 3367-3371 (2014)
- Becker, B. Su, A. Bergmann, K. Weisshart, O. Holub, Simultaneous Fluorescence and Phosphorescence Lifetime Imaging. Proc. SPIE 7903, 790320 (2011)
- Becker, Fluorescence Lifetime Imaging Techniques: Time-correlated single-photon counting. In: L. Marcu. P.M.W. French, D.S. Elson, (eds.), Fluorecence lifetime spectroscopy and imaging. Principles and applications in biomedical diagnostics. CRC Press, Taylor & Francis Group, Boca Raton, London, New York (2015)
- Becker, V. Shcheslavskiy, A. Rück, Simultaneous phosphorescence and fluorescence lifetime imaging by multi-dimensional TCSPC and multi-pulse Excitation. In: R. I. Dmitriev (ed.), Multi-parameteric live cell microscopy of 3D tissue models. Springer (2017)
- Becker, Fluorescence lifetime imaging by multi-dimensional time correlated single photon counting. Medical Photonics 27, 41-61 (2015)
- Becker, Introduction to Multi-Dimensional TCSPC. In W. Becker (ed.) Advanced time-correlated single photon counting applications. Springer, Berlin, Heidelberg, New York (2015)
- Becker & Hickl GmbH, DCS-120 Confocal Scanning FLIM Systems, user handbook. 6th ed. (2015), available on www.becker-hickl.com
- Becker & Hickl GmbH, Modular FLIM Systems for Zeiss LSM 710 / 780 / 880 laser Scanning Microscopes. 6th ed. (2015), available on Becker-Hickl.com
- I. Dmitriev, A. V. Zhdanov, Y. M. Nolan, D. B. Papkovsky, Imaging of neurosphere oxygenation with phosphorescent probes. Biomaterials 34, 9307-9317 (2013)
- I. Dmitriev, A. V. Kondrashina, K. Koren, I. Klimant, A. V. Zhdanov, J. M. P. Pakan, K. W. McDermott, D. B. Papkovsky, Small molecule phosphorescent probes for O2 imaging in 3D tissue models. Biomater. Sci. 2, 853-866 (2014)
- Jenkins, R. I. Dmitriev, D. B. Papkovsky, Imaging Cell and Tissue O2 by TCSPC-PLIM. In: W. Becker (ed.) Advanced time-correlated single photon counting applications. Springer, Berlin, Heidelberg, New York (2015)
- Kalinina, V. Shcheslavskiy, W. Becker, J. Breymayer, P. Schäfer, A. Rück, Correlative NAD(P)H-FLIM and oxygen sensing-PLIM for metabolic mapping. J. Biophotonics 9(8):800-811 (2016)
- I. S. Kritchenkov, V. G. Mikhnevich, V. S. Stashchak, A. I. Solomatina , D. O. Kozina, V, V. Sokolov, S. P. Tunik, Novel NIR-Phosphorescent Ir(III) Complexes: Synthesis, Characterization and Their Exploration as Lifetime-Based O2 Sensors in Living Cells. Molecules 27, 3156 1-18 (2022)
- Kurokawa, H. Ito, M. Inoue, K. Tabata, Y. Sato, K. Yamagata, S. Kizaka-Kondoh, T. Kadonosono, S. Yano, M. Inoue & T. Kamachi, High resolution imaging of intracellular oxygen concentration by phosphorescence lifetime, Scientific Reports 5, 1-13 (2015)
- Lukina, A. Orlova, M. Shirmanova, D. Shirokov, A. Pavlikov, A. Neubauer, H. Studier, W. Becker, E. Zagaynova, T. Yoshihara, S. Tobita, V. Shcheslavskiy, Interrogation of metabolic and oxygen states of tumors with fiber-based luminescence lifetime spectroscopy. Optics Letters 42(4) 731-734 (2017)
- Y. P. Parshina, A. D. Komarova, L. N. Bochkarev, T. A. Kovylina, A. A. Plekhanov, L. G. Klapshina, A. N. Konev, A. M. Mozherov, I.a D. Shchechkin, M. A. Sirotkina, V. I. Shcheslavskiy, M. V. Shirmanova, Simultaneous Probing of Metabolism and Oxygenation of Tumors In Vivo Using FLIM of NAD(P)H and PLIM of a New Polymeric Ir(III) Oxygen Sensor. Int. J. Mol. Sci. 23, 10263 1 -24
- I. Shcheslavskiy, A. Neubauer, R. Bukowiecki, F. Dinter, W. Becker, Combined fluorescence and phosphorescence lifetime imaging. Appl. Phys. Lett. 108, 091111-1 to -5 (2016)
- Shibata, S. Ichioka, J. Ando, A. Kamiya, Microvascular and interstitial PO2 measurement in rat skeletal muscle by phosphorescence quenching. J. Appl. Physiol. 91, 321-327 (2001)
- Toncelli, O. V. Arzhakova, A. Dolgova, A. L. Volynskii, N. F. Bakeev, J. P. Kerry, D. B. Papkovsky, Oxygen-sensitive phosphorescent nanomaterials produced from high density polyethylene films by local solvent-crazing. Anal. Chem. 86(3), 1917-23 (2014)
- V. Zhdanov, A. V. Golubeva, I. A. Okkelman, J. F. Cryan, D. B. Papkovsky, Imaging of oxygen gradients in giant umbrella cells: an ex vivo PLIM study. Am J Physiol Cell Physiol 309: C000–C000, 2015
