Principles
FLIM of Fast Physiological Effects
Physiological effects in live cells and tissues happen at a time scale from milliseconds to tens of seconds. Examples are Ca++ transients in neurons and chlorophyll transients in plants. Multidimensional TCSPC can record such effects by FLITS (Fluorescence-Lifetime Transient Scanning) and Temporal Mosaic Imaging (TMI). FLITS builds up a photon distribution over the times of the photons after the laser pulses, the distance along a line within the sample, and the times after a periodic stimulation. TMI builds up a photon distribution over times of the photons after the laser pulses, the location within a two-dimensional scan, and the times after the stimulation. The principles are shown in the figure below. Both procedures can be performed with standard bh TCSPC FLIM systems.
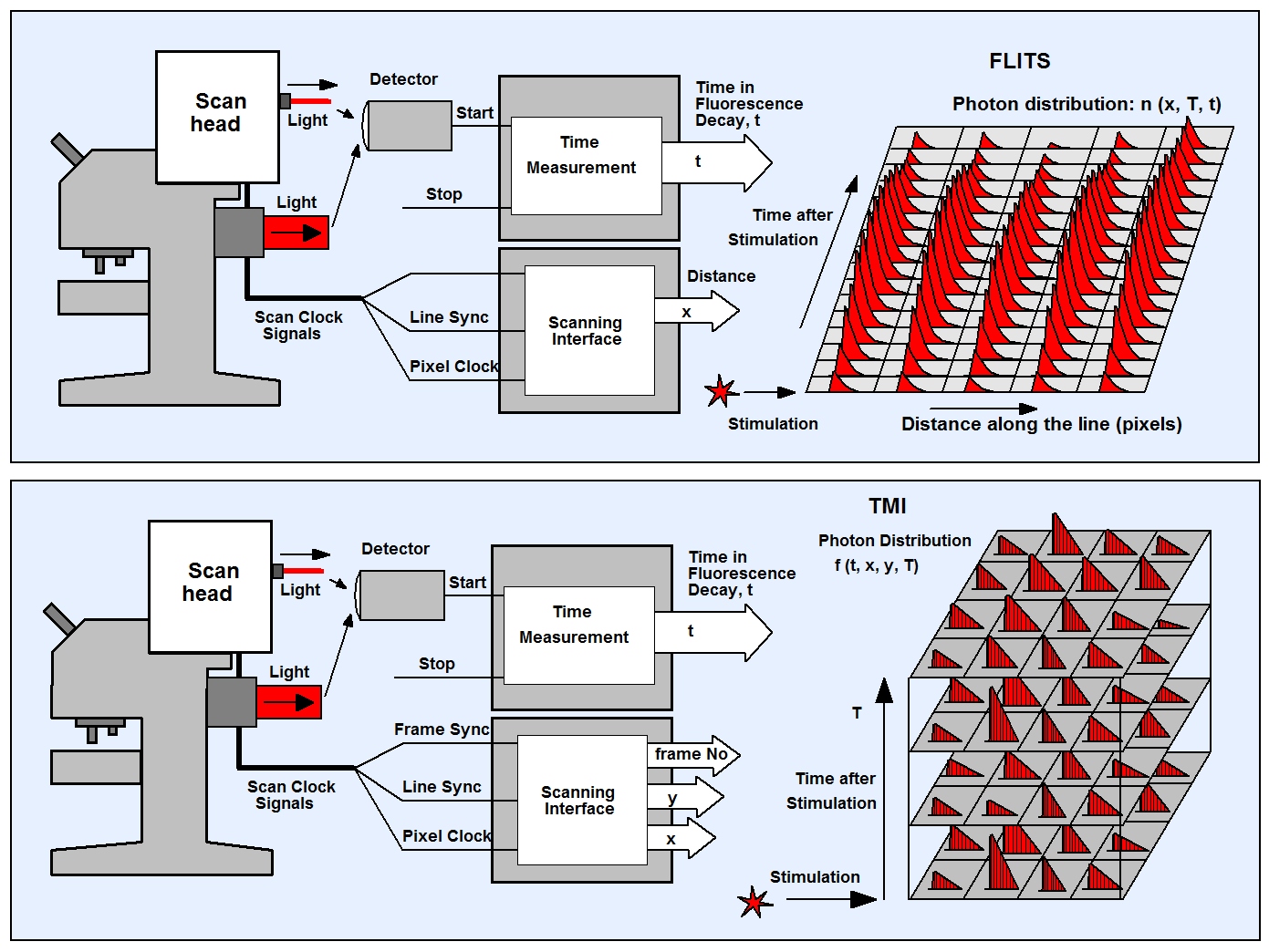
In principle, the photon distributions of FLITS and TMI represent time series of line scans or images. However, compared with a conventional time series, there is a significant difference: FLITS and TMI data can be accumulated. The sample is repeatedly stimulated by an external event, and the start of the FLITS or TMI recording is triggered with the stimulation. With every new stimulation the recording procedure runs through all lines of the FLITS recording or the elements of the TMI mosaic, and accumulates the photons. Accumulation allows data to be recorded without the need of trading photon number and lifetime accuracy against the speed of the time series. Consequently, extremely fast time series can be recorded. In practice, the time per line or mosaic element is only limited by the minimum line or frame time of the scanner.
Calcium++ Transients
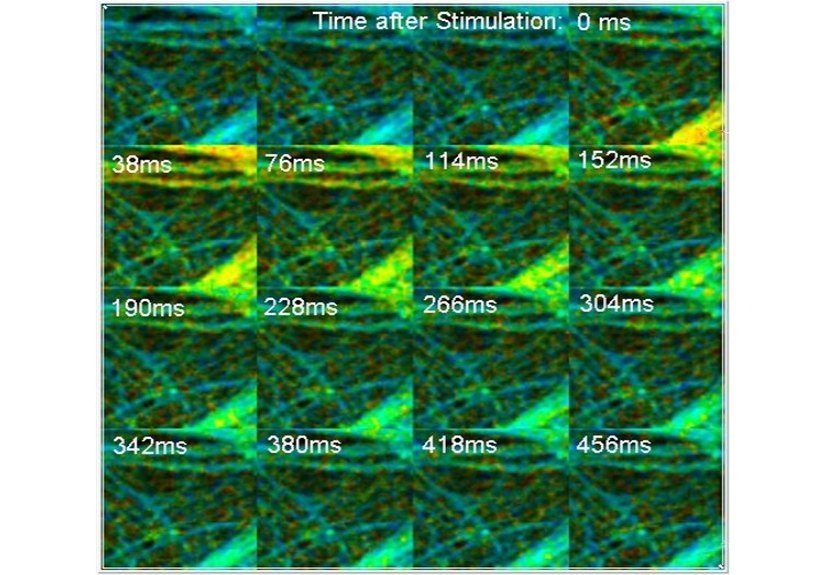
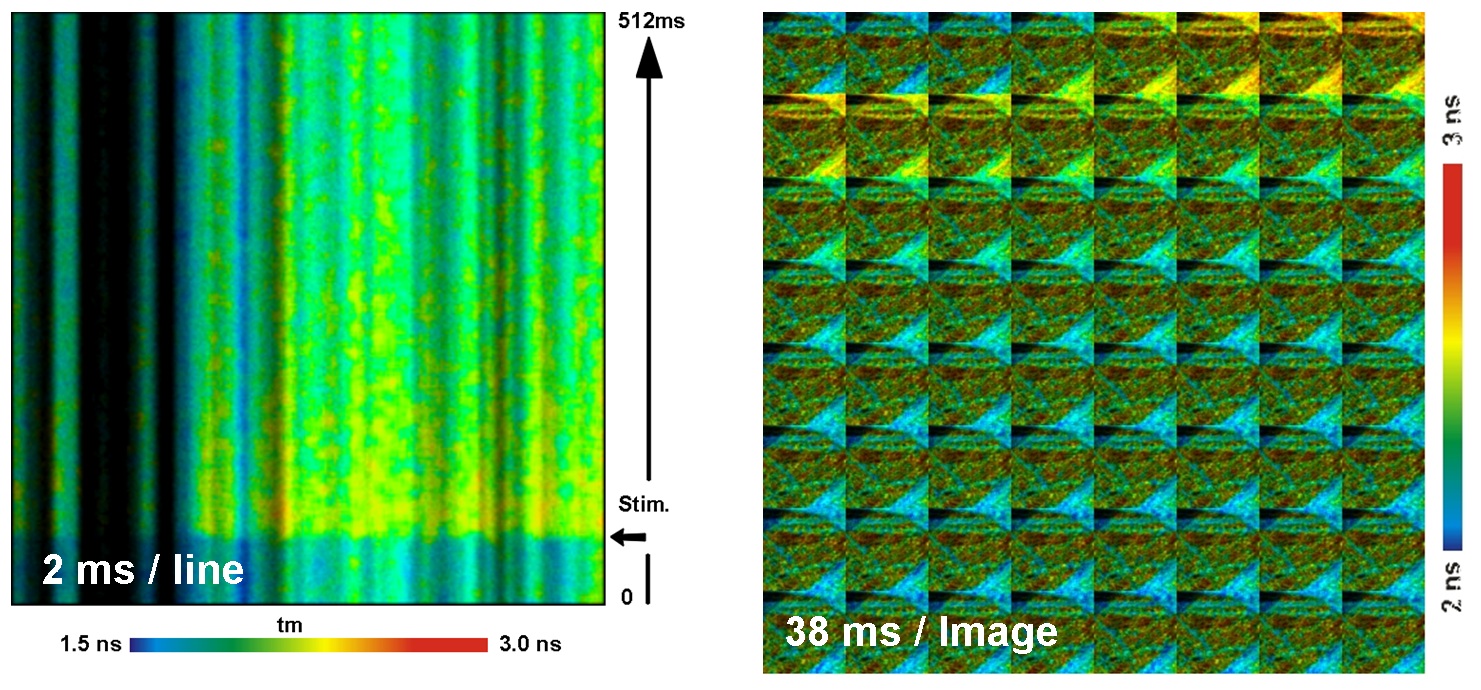
Examples for recording Ca++ transients in cultured neurons are shown in the next figure. The sample was incubated with a calcium sensor (Oregon Green Bapta). The Ca++ transients were stimulated by an electrical pulse periodically applied to the sample. The accumulation of each FLITS or TMI cycle was triggered shortly before the stimulation pulses. The sample was scanned with a line time of 2 ms for FLITS and with a frame time of 38 ms for TMI.
For details please see:
- Becker, The bh TCSPC Handbook, chapter ‘Time-Series FLIM’
- Becker, S. Frere, I. Slutsky, Recording Ca++ Transients in Neurons by TCSPC FLIM. In:F.-J. Kao, G. Keiser, A. Gogoi, (eds.), Advanced optical methods of brain imaging. Springer (2019)
References
- Becker, V. Shcheslavkiy, S. Frere, I. Slutsky, Spatially Resolved Recording of Transient Fluorescence-Lifetime Effects by Line-Scanning TCSPC. Microsc. Res. Techn. 77, 216-224 (2014)
- Becker, The bh TCSPC Handbook 10th ed. (2023), chapter ‘Time-Series FLIM’
- Becker, S. Frere, I. Slutsky, Recording Ca++ Transients in Neurons by TCSPC FLIM. In:F.-J. Kao, G. Keiser, A. Gogoi, (eds.), Advanced optical methods of brain imaging. Springer (2019)
- Frere, I. Slutsky, Calcium imaging using Transient Fluorescence-Lifetime Imaging by Line-Scanning TCSPC. In: W. Becker (ed.) Advanced time-correlated single photon counting applications. Springer, Berlin, Heidelberg, New York (2015)
