Principles
Simultaneous FLIM / PLIM
There is a number of radiative relaxation mechanisms which occur on a much longer time scale than fluorescence. The commonly known one is phosphorescence, i.e. emission from the triplet state of organic dyes. Phosphorescence is usually weak at room temperature. However, strong phosphorescence emission is obtained for organic complexes of ruthenium, platinum, terbium, and palladium. Of special interest for live-cell imaging is that phosphorescence is strongly quenched by oxygen. The phosphorescence lifetime is therefore used as a sensing function for the local oxygen concentration, or oxygen partial pressure, pO2.
Oxygen measurement is especially important for metabolic imaging. The metabolic state of cancer cells and normal cells is different, but it also depends on the availability of oxygen. pO2 measurement therefore has to be performed simultaneously with NAD(P)H FLIM.
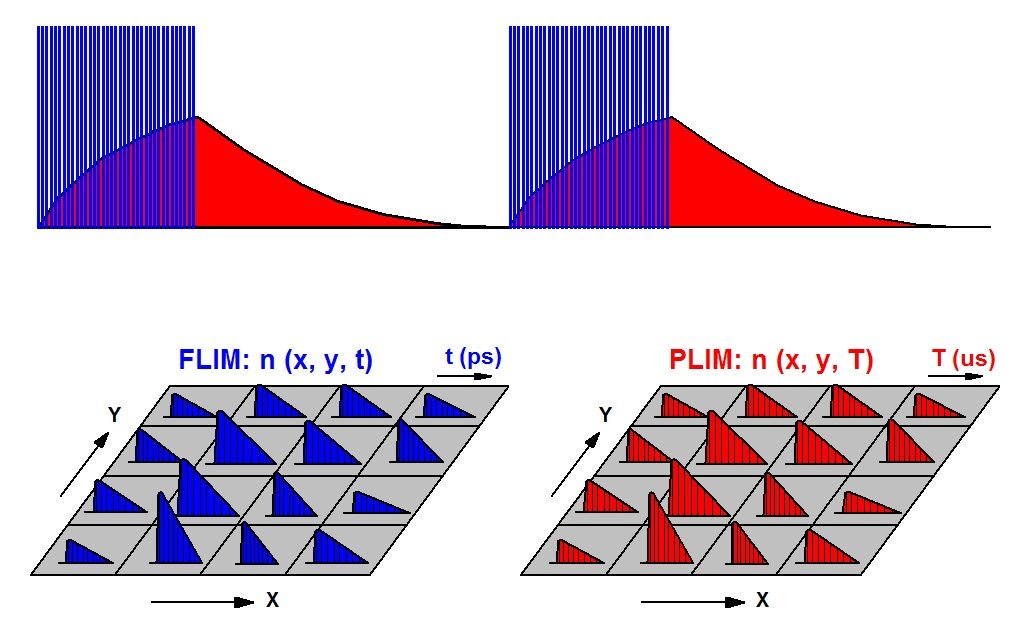
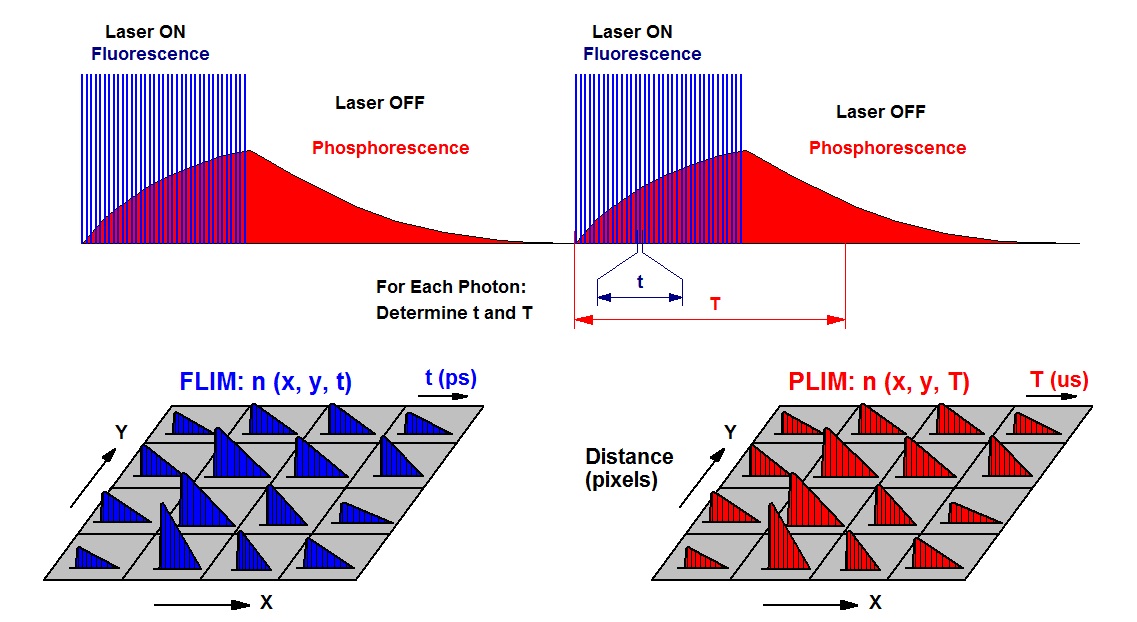
The task is solved by modulating the excitation laser of a FLIM system at a period in the microsecond of millisecond range. A FLIM image is obtained from the photon times in the laser pulse period, a FLIM image from the times in the modulation period. The principle is shown in the figure below.
Implementation
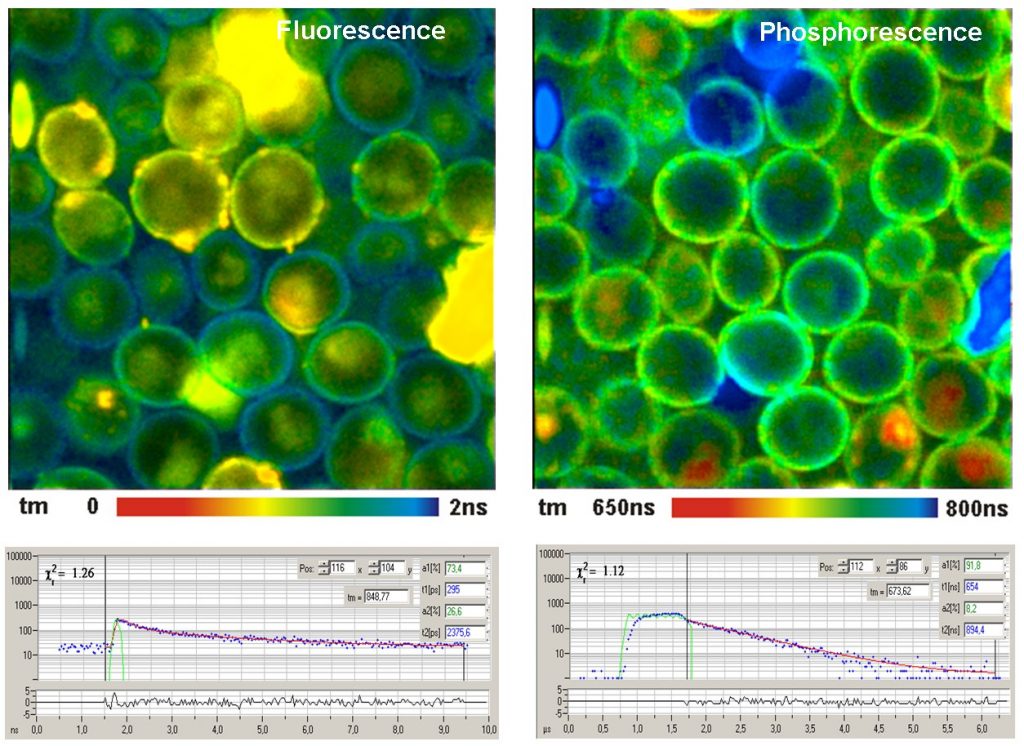
FLIM / PLIM is fully implemented in the bh DCS-120 confocal and multiphoton FLIM systems. It can be applied to other laser scanning microscopes as well, provides there is a way to modulate the excitation laser(s). An example of a FLIM/PLIM result is shown in the figure below.
For details and more references please see:
Application note ‘Simultaneous Phosphorescence and Fluorescence Lifetime Imaging by Multi-Dimensional TCSPC and Multi-Pulse Excitation‘
References
References for Simultaneous FLIM / PLIM
- Baggaley, S. W. Botchway, J. W. Haycock, H. Morris, I. V. Sazanovich, J. A. G. Williams, J. A. Weinstein, Long-lived metal complexes open up microsecond lifetime imaging microscopy under multiphoton excitation: from FLIM to PLIM and beyond. Chem. Sci. 5, 879-886 (2014)
- Baggaley, M. R. Gill, N. H. Green, D. Turton, I. V. Sazanovich, S. W. Botchway, C. Smythe, J. W. Haycock, J. A. Weinstein, J. A. Thomas, Dinuclear Ruthenium(II) Complexes as Two-Photon, Time-Resolved Emission Microscopy Probes for Cellular DNA. Angew. Chem. Int. Ed. Engl. 53, 3367-3371 (2014)
- Becker, V. Shcheslavskiy, A. Rück, Simultaneous phosphorescence and fluorescence lifetime imaging by multi-dimensional TCSPC and multi-pulse Excitation. In: R. I. Dmitriev (ed.), Multi-parameteric live cell microscopy of 3D tissue models. Springer (2017)
- Becker, B. Su, A. Bergmann, K. Weisshart, O. Holub, Simultaneous Fluorescence and Phosphorescence Lifetime Imaging. Proc. SPIE 7903, 790320 (2011)
- Becker, Fluorescence Lifetime Imaging Techniques: Time-correlated single-photon counting. In: L. Marcu. P.M.W. French, D.S. Elson, (eds.), Fluorecence lifetime spectroscopy and imaging. Principles and applications in biomedical diagnostics. CRC Press, Taylor & Francis Group, Boca Raton, London, New York (2015)
- Becker, Fluorescence lifetime imaging by multi-dimensional time correlated single photon counting. Medical Photonics 27, 41-61 (2015)
- Becker, Introduction to Multi-Dimensional TCSPC. In W. Becker (ed.) Advanced time-correlated single photon counting applications. Springer, Berlin, Heidelberg, New York (2015)
- Becker & Hickl GmbH, DCS-120 Confocal Scanning FLIM Systems, user handbook. 6th ed. (2015), available on www.becker-hickl.com
- Becker & Hickl GmbH, Modular FLIM Systems for Zeiss LSM 710 / 780 / 880 laser Scanning Microscopes. 6th ed. (2015), available on Becker-Hickl.com
- I. Dmitriev, A. V. Zhdanov, Y. M. Nolan, D. B. Papkovsky, Imaging of neurosphere oxygenation with phosphorescent probes. Biomaterials 34, 9307-9317 (2013)
- I. Dmitriev, A. V. Kondrashina, K. Koren, I. Klimant, A. V. Zhdanov, J. M. P. Pakan, K. W. McDermott, D. B. Papkovsky, Small molecule phosphorescent probes for O2 imaging in 3D tissue models. Biomater. Sci. 2, 853-866 (2014)
- Jenkins, R. I. Dmitriev, D. B. Papkovsky, Imaging Cell and Tissue O2 by TCSPC-PLIM. In: W. Becker (ed.) Advanced time-correlated single photon counting applications. Springer, Berlin, Heidelberg, New York (2015)
- Kalinina, V. Shcheslavskiy, W. Becker, J. Breymayer, P. Schäfer, A. Rück, Correlative NAD(P)H-FLIM and oxygen sensing-PLIM for metabolic mapping. J. Biophotonics 9(8):800-811 (2016)
- Kurokawa, H. Ito, M. Inoue, K. Tabata, Y. Sato, K. Yamagata, S. Kizaka-Kondoh, T. Kadonosono, S. Yano, M. Inoue & T. Kamachi, High resolution imaging of intracellular oxygen concentration by phosphorescence lifetime, Scientific Reports 5, 1-13 (2015)
- Lukina, A. Orlova, M. Shirmanova, D. Shirokov, A. Pavlikov, A. Neubauer, H. Studier, W. Becker, E. Zagaynova, T. Yoshihara, S. Tobita, V. Shcheslavskiy, Interrogation of metabolic and oxygen states of tumors with fiber-based luminescence lifetime spectroscopy. Optics Letters 42(4) 731-734 (2017)
- I. Shcheslavskiy, A. Neubauer, R. Bukowiecki, F. Dinter, W. Becker, Combined fluorescence and phosphorescence lifetime imaging. Appl. Phys. Lett. 108, 091111-1 to -5 (2016)
- Shibata, S. Ichioka, J. Ando, A. Kamiya, Microvascular and interstitial PO2 measurement in rat skeletal muscle by phosphorescence quenching. J. Appl. Physiol. 91, 321-327 (2001)
- Toncelli, O. V. Arzhakova, A. Dolgova, A. L. Volynskii, N. F. Bakeev, J. P. Kerry, D. B. Papkovsky, Oxygen-sensitive phosphorescent nanomaterials produced from high density polyethylene films by local solvent-crazing. Anal. Chem. 86(3), 1917-23 (2014)
- V. Zhdanov, A. V. Golubeva, I. A. Okkelman, J. F. Cryan, D. B. Papkovsky, Imaging of oxygen gradients in giant umbrella cells: an ex vivo PLIM study. Am J Physiol Cell Physiol 309: C000–C000, 2015
